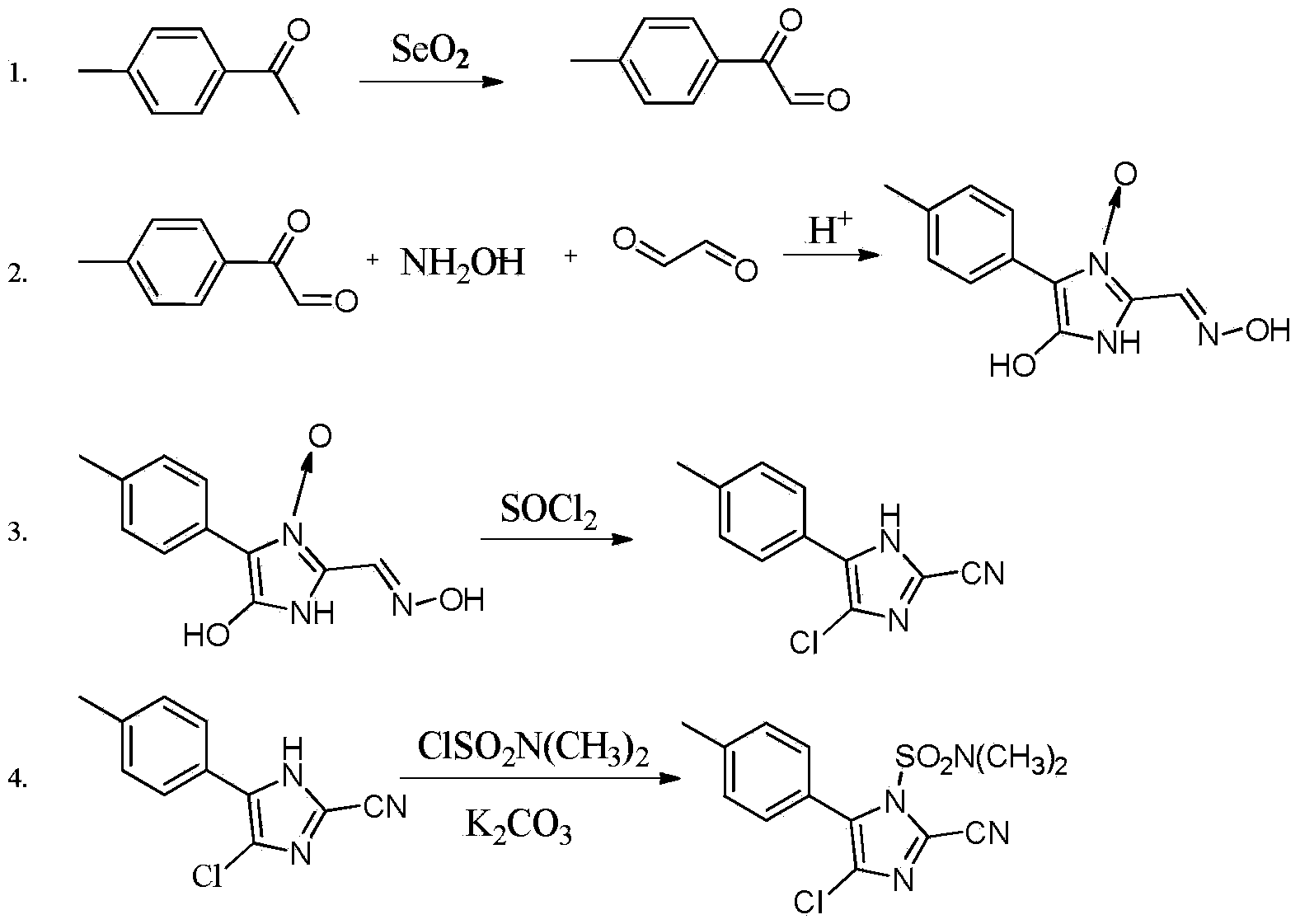
Synthesis method of 4-chloro-2-cyano-N,N-dimethyl-5-(4-methylphenyl)-1H-imidazole-1-sulfonamide
A kind of technology of oximinylmethine imidazole and methyl phenyl, which is applied in 4-chloro-2-cyano-N,N-dimethyl-5-(4-methylphenyl)-1H-imidazole- In the field of synthesis of 1-sulfonamide, it can solve the problems of complex post-treatment process, severe exotherm, and long reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] 1) Synthesis of 2-carbonyl-2-p-benzyl acetaldehyde
[0014] Add 36.6g (0.33mol) of selenium dioxide, 150ml of dioxane, and 10ml of distilled water into a 250ml four-neck flask equipped with a thermometer, a stirrer and a reflux condenser, stir and heat up to 50°C, add 40.4g (0.3mol) For p-methylacetophenone, heat up to 110°C for 5 hours, cool to room temperature, remove the filter residue by filtration, concentrate the filtrate to dryness under reduced pressure, add 80ml of petroleum ether for beating, filter to obtain a reddish-brown solid, use petroleum ether:ethyl acetate= 10 (volume ratio) recrystallized to obtain a purity of 98.5% and a yield of 82.3%.
[0015] 2) Synthesis of 4-hydroxy-4(5)-(4-methylphenyl)-2-oximinomethymidazolium-3-oxo
[0016] Add 44.4g (0.3mol) 2-carbonyl-2-p-benzyl acetaldehyde, 104.3g (1.50mol) hydroxylamine hydrochloride, 200ml methanol and 100ml of water, heated to reflux, reacted for 2 hours, then added 47.9g (0.33mol) 40% glyoxal aqueo...
Embodiment 2
[0028] 1) Synthesis of 2-carbonyl-2-p-benzyl acetaldehyde
[0029] Add 50.0g (0.45mol) of selenium dioxide, 150ml of dioxane, and 10ml of distilled water into a 250ml four-neck flask equipped with a thermometer, a stirrer and a reflux condenser, heat up to 50°C under vigorous stirring, and add 40.4g (0.3mol) mol) p-methyl acetophenone, warming up to 110°C for 5 hours reaction, then cooling the reaction solution to room temperature, filtering to remove the selenium generated, concentrating the filtrate to dryness under reduced pressure, adding 80ml of petroleum ether for beating, and filtering to obtain a reddish-brown solid, Recrystallized with petroleum ether: ethyl acetate = 10 (volume ratio), the purity was 98.5%, and the yield was 83.2%.
[0030] 2) Synthesis of 4-hydroxy-4(5)-(4-methylphenyl)-2-oximinomethymidazolium-3-oxo
[0031] Add 44.4g (0.3mol) 2-carbonyl-2-p-benzyl acetaldehyde, 104.3g (1.50mol) hydroxylamine sulfate, 200ml methanol and 100ml of water, heated to ...
Embodiment 3
[0038] The method is basically the same as in Example 2, except that the solvent in step 3) is replaced by dichloroethane and N,N-dimethylformamide. Based on p-methyl acetophenone, the total yield of cyafamid was 45.7%, and the purity was 89.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com