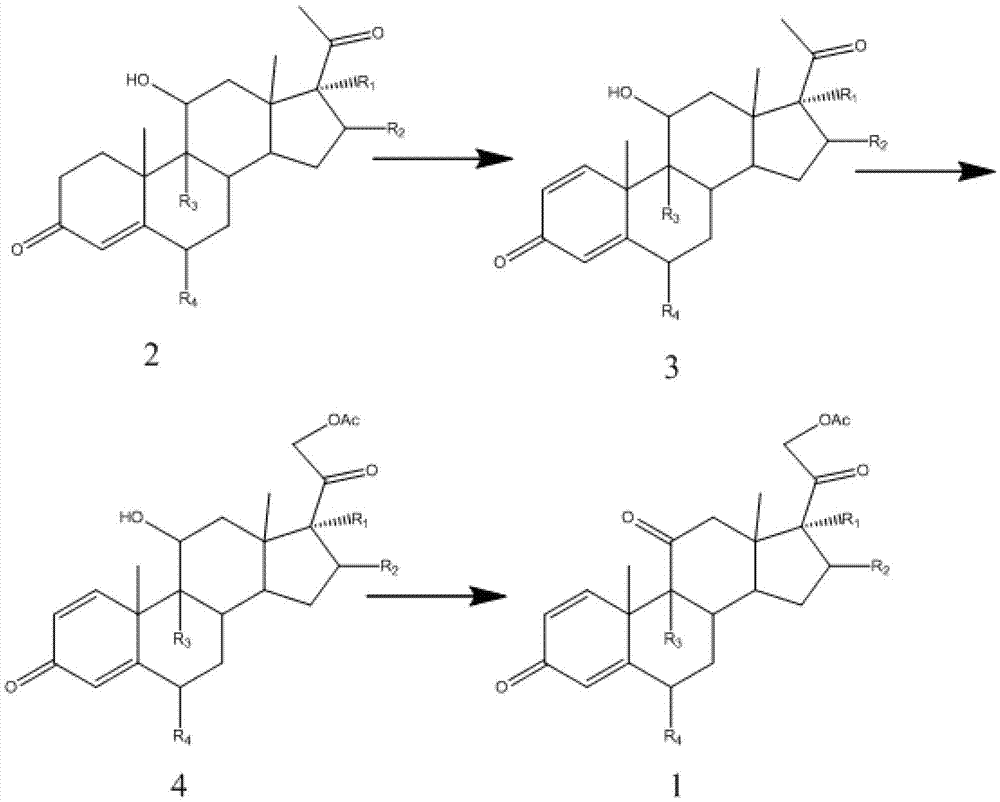
Preparation method of progestin-1,4-double bond-11-one-21-acetate compound
A compound, iodide technology, applied in the field of preparation of progesterone compounds, can solve the problems of small progress and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
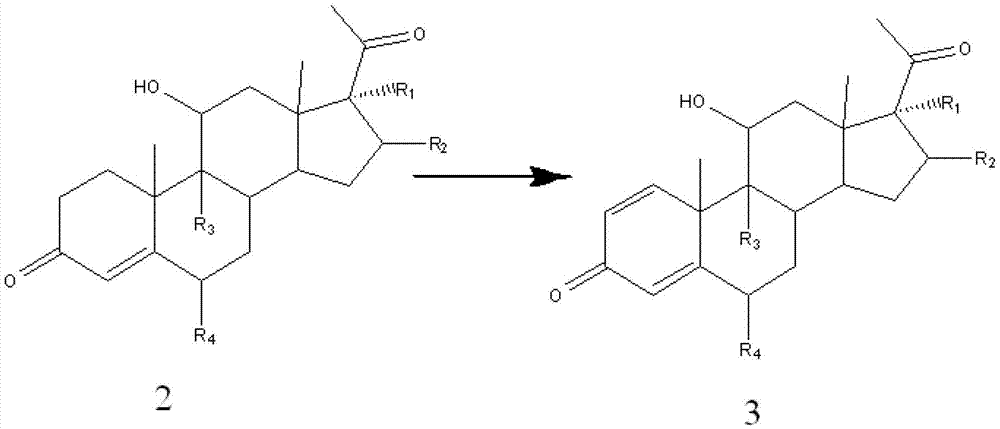
[0073] General reaction formula:
[0074]
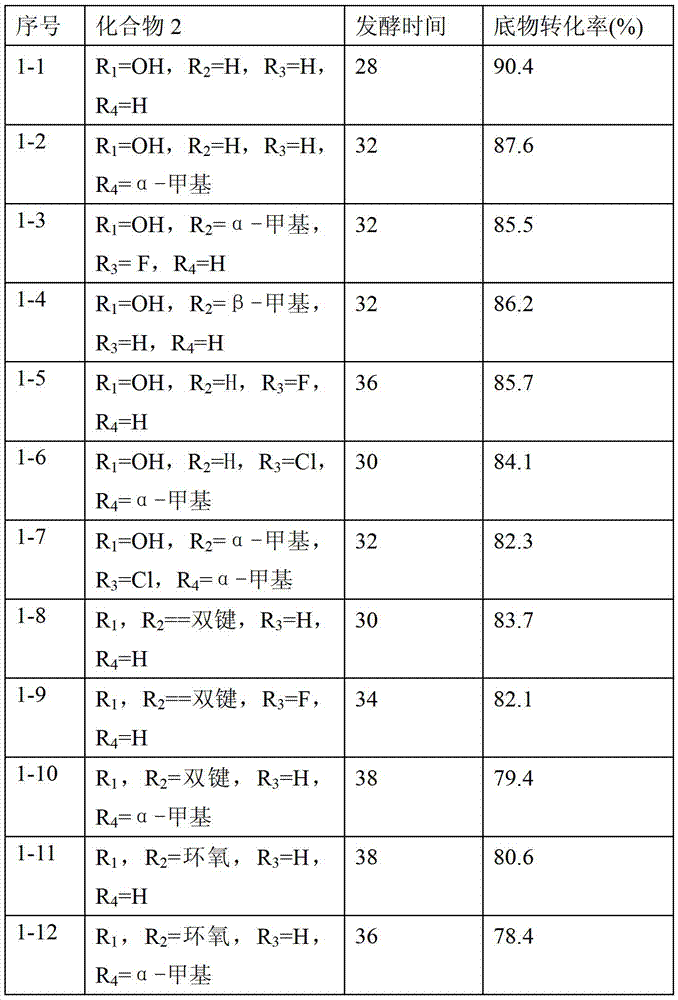
[0075] Reaction process: Arthrobacter Simple ATCC 21032 was subjected to slant culture, primary culture and secondary culture in sequence, the culture temperature was 28-32°C, the micronized substrate compound 2 was put into a 5L fermenter, and the feeding concentration was 3 -4%, the reaction temperature is 28-32°C, when the remaining substrate is less than 4%, the fermentation is considered to be complete. After the reaction was completed, the temperature was raised to 70°C to terminate the reaction, the fermentation broth was extracted with ethyl acetate, the ethyl acetate was concentrated, and the conversion rate of the substrate was measured, and compound 3 was obtained by recrystallization method using methanol.
[0076]
Embodiment 2
[0082] General reaction formula:
[0083]
[0084] Synthesis:
[0085] Put 5g of compound 3 into the reactor, add 50ml of methanol, cool down to 20°C, add 3g of calcium oxide, and add iodine solution dropwise for 1-2 hours (preparation method of iodine solution: put 9g of iodine into a 200ml single-necked bottle, add 2g of anhydrous chlorine Calcium chloride, 30ml methanol, stirring for 2 hours to dissolve) is completed, keep warm until there is no raw material point, add 400ml of ice water, stir to precipitate solid, filter to obtain iodide, no need to dry, then put it directly into another reactor, add 200ml Acetone, 20ml of glacial acetic acid, 10g of potassium acetate, heated to 60°C and reacted to the point of no raw materials, concentrated acetone under reduced pressure, added 400ml of ice water to stir the precipitate, filtered to obtain a solid, and dried at 80°C to obtain compound 4.
[0086]
[0087]
Embodiment 3
[0089] General reaction formula:
[0090]
[0091] Reaction process: add 10g compound 4 (content is 97.4%, maximum impurity <2%), dimethyl sulfoxide 30g, activator to react in 100ml chloroalkane at -15 ℃, detect substrate content by high performance liquid phase< After 1%, add an organic base to adjust the pH to 8.5-10, concentrate under reduced pressure to no chlorinated alkanes, cool down to 0°C, dilute with 100ml of 0°C water under stirring, and filter to obtain compound 1.
[0092]
[0093]
[0094]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com