DDP-4 inhibitor drug orally disintegrating tablets and preparing method
A technology of DDP-4 and inhibitors, which is applied in drug combination, drug delivery, and pharmaceutical formulation, etc. It can solve problems such as uneven drug content and increased impurities in tablets, and achieve good taste, good uniformity, and smooth surface shape.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Alogliptin Benzoate 34g
[0034] Lactose 48.5g
[0035] 30g pregelatinized starch
[0036] Microcrystalline Cellulose 18g
[0037] L-Hydroxypropylcellulose 15g
[0038] Cyclamate 1.5g
[0039] Magnesium Stearate 1.5g
[0040] Micronized silica gel 1.5 g
[0041] Makes 1000 pieces
[0042] Preparation process: mix the prescription amount of lactose, pregelatinized starch, microcrystalline cellulose, L-hydroxypropyl cellulose, cyclamate and micropowder silica gel in equal increments, and then add the prescription amount of agrelide benzoate Alogliptin and the above-mentioned mixed powder are mixed uniformly in equal increments, and finally the prescribed amount of magnesium stearate is added and mixed uniformly, the weight and hardness of the tablet are determined, and the tablets are directly compressed to obtain alogliptin orally disintegrating tablets.
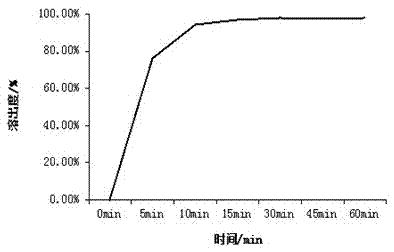
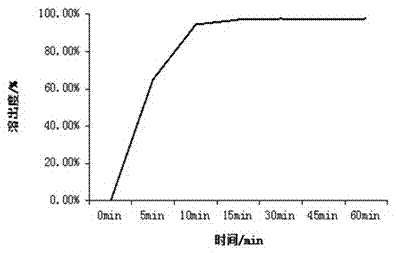
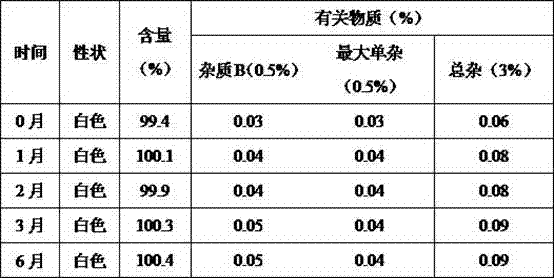
[0043] Appearance: The orally disintegrating tablets of alogliptin prepared by this method are off-white with...
Embodiment 2
[0049] Alogliptin Benzoate 17g
[0050] Lactose 65.5g
[0051] 30g pregelatinized starch
[0052] Microcrystalline Cellulose 18g
[0053] L-Hydroxypropylcellulose 15g
[0054] Cyclamate 1.5g
[0055] Magnesium Stearate 1.5g
[0056] Micronized silica gel 1.5 g
[0057] Makes 1000 pieces
[0058] Preparation process: mix the prescription amount of lactose, pregelatinized starch, microcrystalline cellulose, L-hydroxypropyl cellulose, cyclamate and micropowder silica gel in equal increments, and then add the prescription amount of agrelide benzoate Alogliptin and the above-mentioned mixed powder are mixed uniformly in equal increments, and finally the prescribed amount of magnesium stearate is added and mixed uniformly, the weight and hardness of the tablet are determined, and the tablets are directly compressed to obtain alogliptin orally disintegrating tablets.
[0059] Appearance: The orally disintegrating tablets of alogliptin prepared by this method are off-white with...
Embodiment 3
[0065] Alogliptin Benzoate 8.5g
[0066] Lactose 74g
[0067] 30g pregelatinized starch
[0068] Microcrystalline Cellulose 18g
[0069] L-Hydroxypropylcellulose 15g
[0070] Cyclamate 1.5g
[0071] Magnesium Stearate 1.5g
[0072] Micronized silica gel 1.5 g
[0073] Makes 1000 pieces
[0074] Preparation process: mix the prescription amount of lactose, pregelatinized starch, microcrystalline cellulose, L-hydroxypropyl cellulose, cyclamate and micropowder silica gel in equal increments, and then add the prescription amount of agrelide benzoate Alogliptin and the above-mentioned mixed powder are mixed uniformly in equal increments, and finally the prescribed amount of magnesium stearate is added and mixed uniformly, the weight and hardness of the tablet are determined, and the tablets are directly compressed to obtain alogliptin orally disintegrating tablets.
[0075] Appearance: The orally disintegrating tablets of alogliptin prepared by this method are off-white with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com