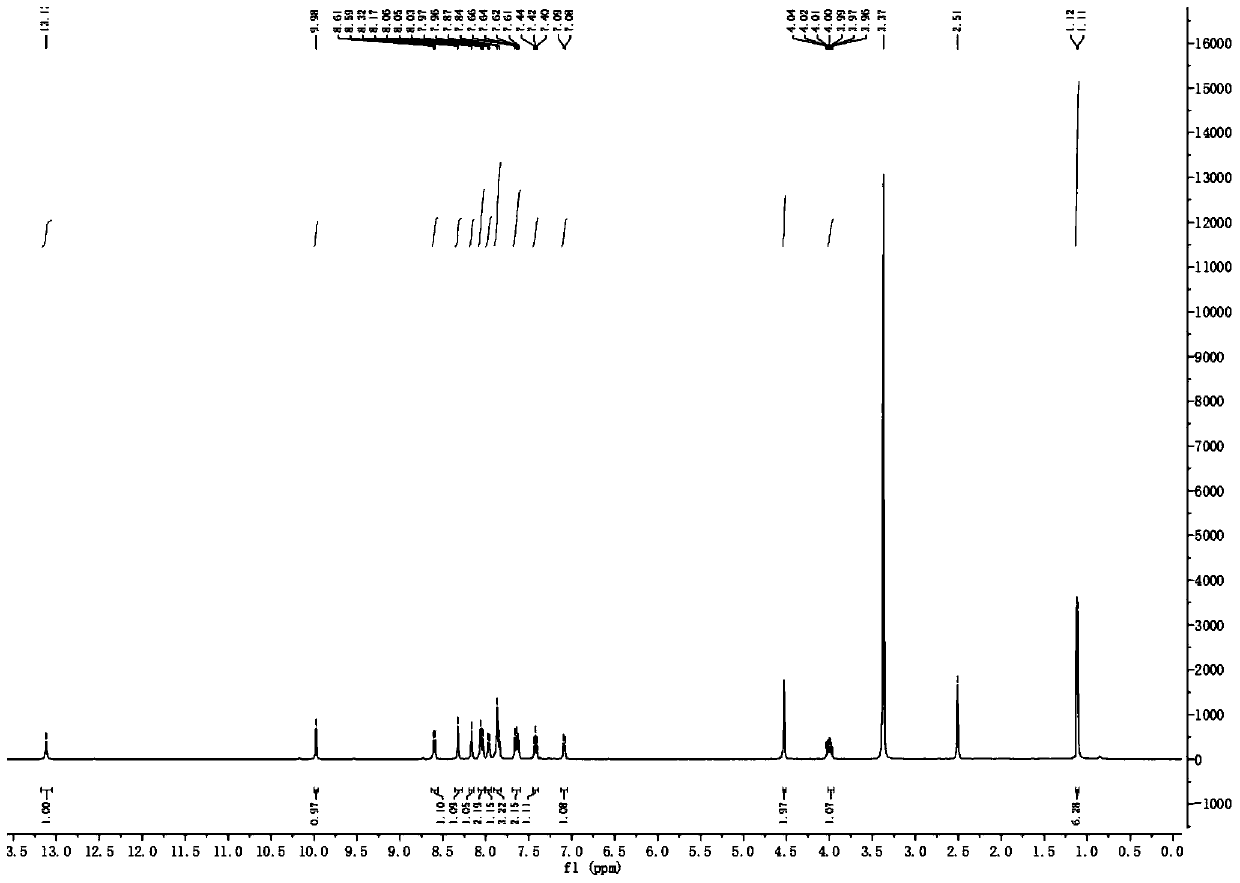
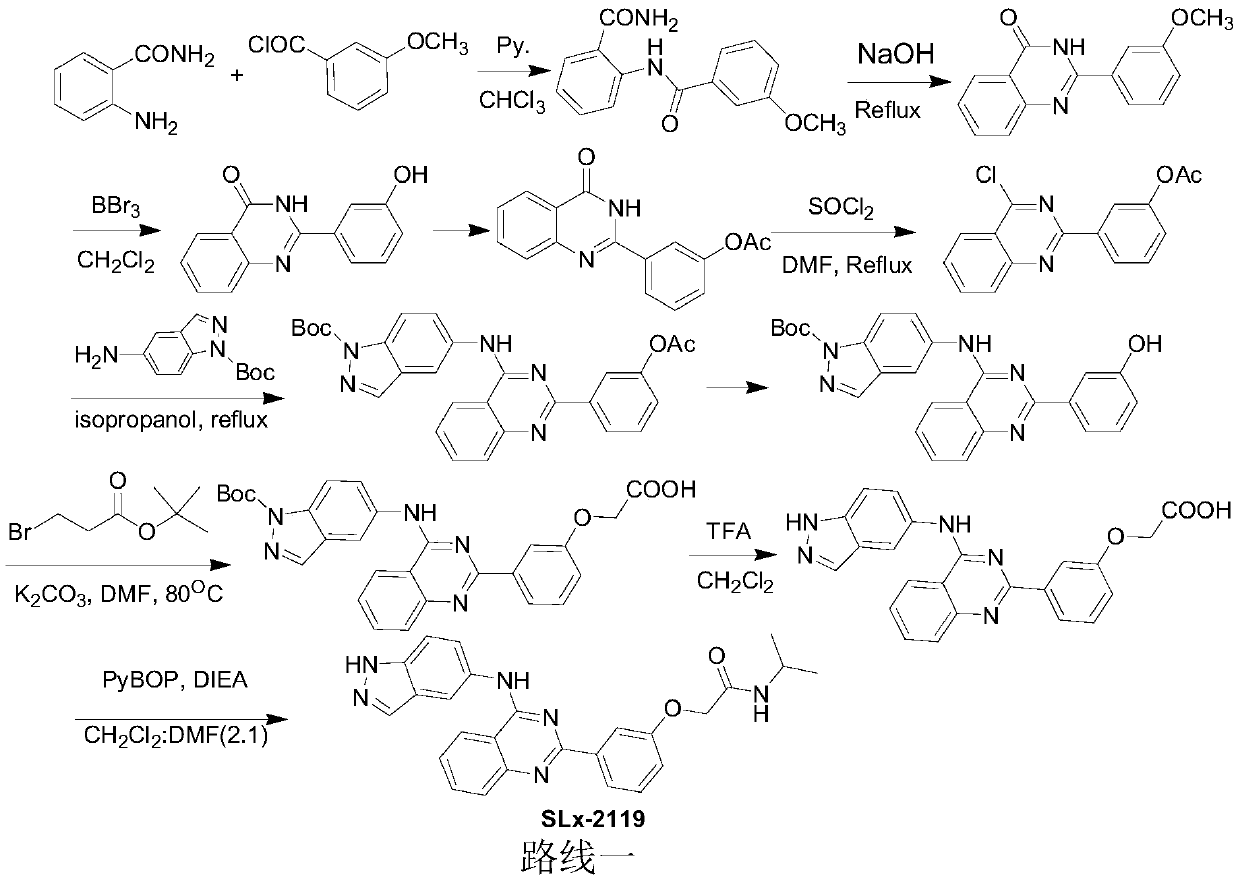
The synthetic method of slx-2119
A technology of slx-2119 and a synthesis method, which is applied in the synthesis field of SLx-2119, can solve the problems of high cost, unfriendly environment, high price and the like, and achieves the effects of low cost, environmental friendliness and short reaction route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Preparation of 2-bromo-N-isopropylacetamide I:
[0037]
[0038] In a 25mL round bottom flask, add dichloromethane (5mL) and isopropylamine (1.2mmol) successively, plug a rubber stopper, and protect with nitrogen; under stirring in an ice-water bath, bromoacetyl bromide (1mmol) After dropping, turn to room temperature and stir after 5 minutes, and detect the reaction by TLC after 1 hour. After the bromoacetyl bromide disappears, stop stirring, filter off the white solid, and wash with dichloromethane, add dilute hydrochloric acid solution to the filtrate for washing, two The organic layer was extracted with methyl chloride, washed with saturated brine, anhydrous Na 2 SO 4 Drying, spin-drying, and column chromatography gave 2-bromo-N-isopropylacetamide I as a white solid (yield 71%), 1 H NMR (500MHz, CDCl 3 ) δ (ppm): 6.39 (br, NH, 1H), 4.11-4.04 (m, 1H), 3.84 (s, 2H), 1.19 (d, J=6.0Hz, 6H).
[0039] (2) Preparation of 3-(isopropylcarbamoyl-methoxy)-methyl ben...
Embodiment 2
[0058] (1) Preparation of 2-bromo-N-isopropylacetamide Ⅰ: Add dichloromethane (1mL) and isopropylamine (1mmol) successively in a 25mL round bottom flask, put on a rubber stopper, and protect under nitrogen; Under stirring in an ice-water bath, drop bromoacetyl bromide (1 mmol) within 2 minutes. After 5 minutes, stir at room temperature. After 1 hour, the reaction was detected by TLC. After the bromoacetyl bromide disappeared, stop stirring, filter off the white solid, and use Washing with dichloromethane, adding dilute hydrochloric acid solution to the filtrate for washing, extracting the organic layer with dichloromethane, washing with saturated brine, anhydrous Na 2 SO 4 Drying, spin-drying, and column chromatography gave 2-bromo-N-isopropylacetamide I as a white solid (68% yield).
[0059] (2) Preparation of 3-(isopropylcarbamoyl-methoxy)-methyl benzoate II: In a 25mL round bottom flask, add DMF (1.0mL), 2-bromo-N-isopropyl Acetamide I (1mmol), methyl 3-hydroxybenzoate (1...
Embodiment 3
[0066] (1) Preparation of 2-bromo-N-isopropylacetamide I: Add dichloromethane (10mL) and isopropylamine (1.5mmol) sequentially into a 25mL round bottom flask, plug the rubber stopper, and insert nitrogen gas Under stirring in an ice-water bath, drop bromoacetyl bromide (1mmol) within 2 minutes, and then stir at room temperature after 5 minutes. After 1 hour, the reaction was detected by TLC. After the bromoacetyl bromide disappeared, stop stirring and filter off the white solid. , and washed with dichloromethane, adding dilute hydrochloric acid solution to the filtrate for washing, dichloromethane extracted the organic layer, washed with saturated brine, anhydrous Na 2 SO 4 Drying, spin-drying, and column chromatography gave 2-bromo-N-isopropylacetamide I as a white solid (yield 71%).
[0067] (2) Preparation of 3-(isopropylcarbamoyl-methoxy)-methyl benzoate II: In a 25mL round bottom flask, add DMF (10.0mL), 2-bromo-N-isopropyl Acetamide Ⅰ (1mmol), methyl 3-hydroxybenzoate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com