Disubstituted derivative of carboline, and preparation method and application thereof
A derivative, carboline II technology, applied in the field of organic electroluminescent materials, can solve problems such as unbalanced electron and hole transport capabilities, low luminous efficiency, and affecting the normal use of carboline derivatives, and achieve high triplet energy , high luminous efficiency and balanced transmission capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
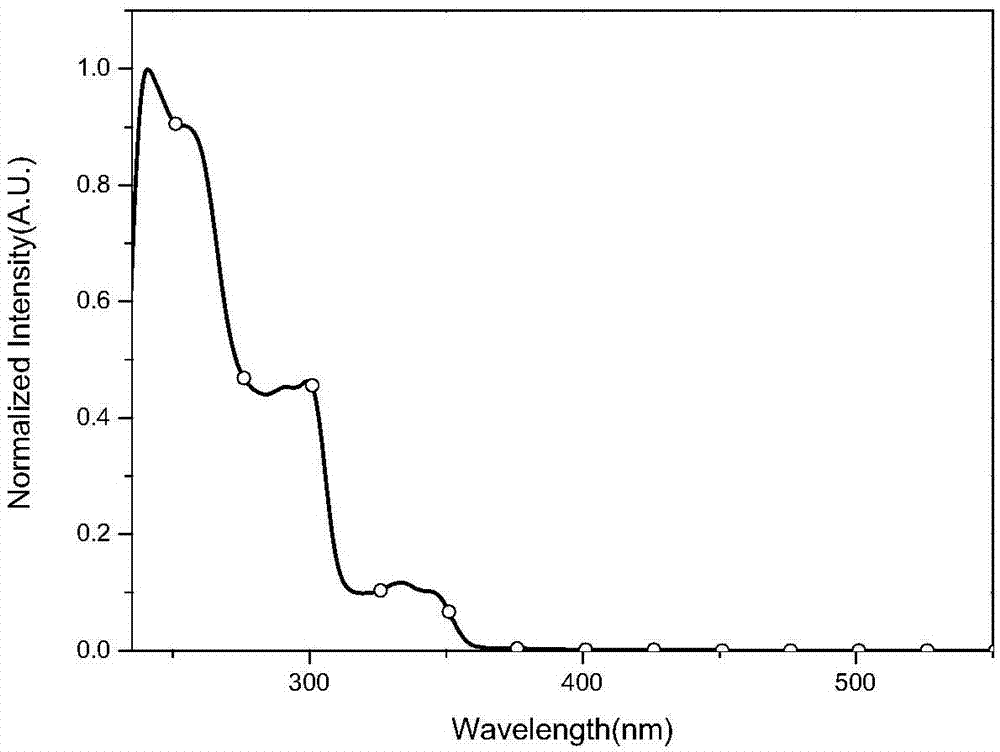
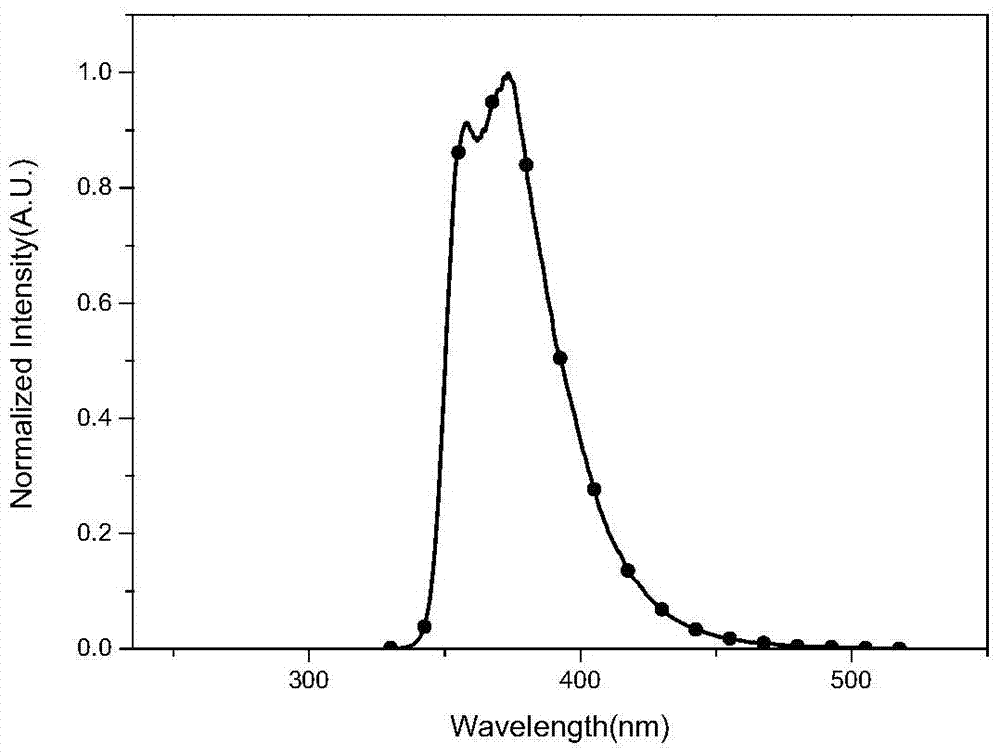
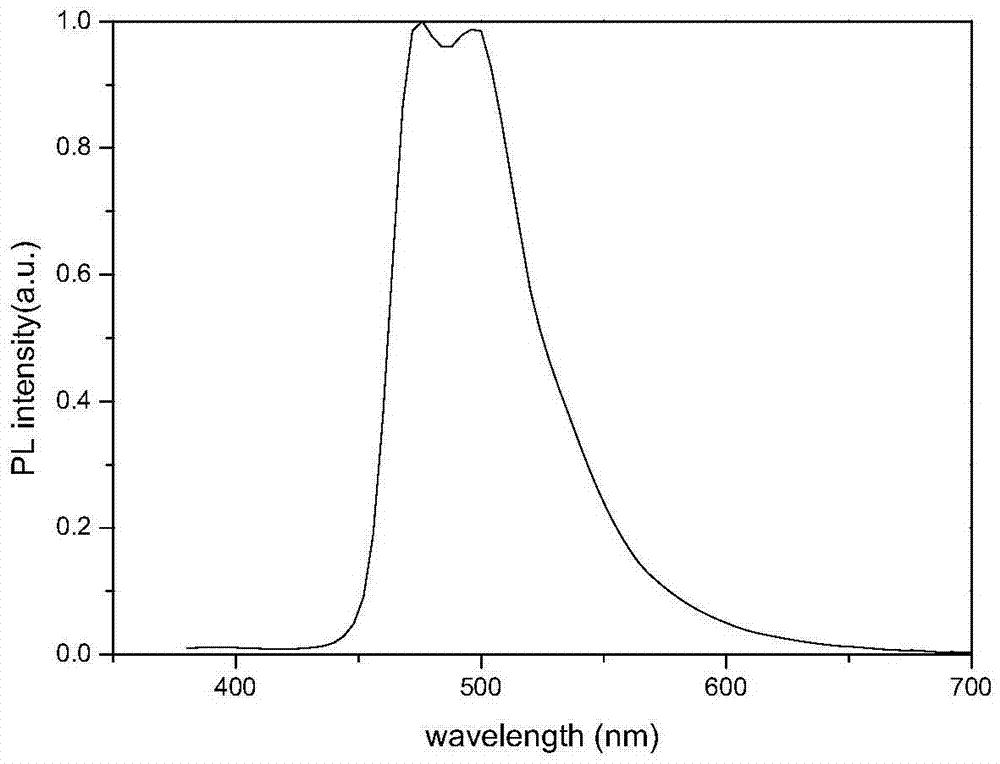
Image
Examples
Embodiment 1
[0067] Embodiment one: the preparation of compound A-5
[0068]a) Synthesis of 2-phenyl-3-nitropyridine (a1): 5 gram 2-chloro-3-nitropyridine, 4.7 gram phenylboronic acid, 8.7 gram potassium carbonate, 0.1 gram palladium acetate, 0.4 gram triphenyl Base phosphine, 60 ml of 1,4-dioxane / distilled water (3:1) was added into a three-necked flask, and stirred under reflux for 24 hours under the protection of nitrogen. After the reaction was completed, distilled water was added to the system and stirred until the solution became clear, then extracted with ethyl acetate, washed with saturated brine, dried, concentrated, and column chromatographed to obtain 6.0 g of solid, with a yield of 93%. Mass spectrum: theoretical value m / e, 190.1; found value 190.1. 1 H-NMR (500MHz, CDCl 3 ): 8.83(1H), 8.11(1H), 7.60-7.54(2H), 7.50-7.43(3H), 7.39(1H).
[0069]
[0070] b) Synthesis of δ-carboline (a2): 5 g of 2-phenyl-3-nitropyridine was dissolved in 30 ml of triethyl phosphite, and stirr...
Embodiment 2
[0081] Embodiment two: the preparation of compound A-6
[0082] 3-(δ-carbolinyl)-phenylboronic acid pinacol ester (a4) 1g, N-(3-bromophenyl)-δ-carboline (a3) 0.9g, potassium carbonate 1.5g, distilled water 20ml , toluene 50ml, ethanol 10ml, palladium acetate 0.1g, triphenylphosphine 0.4g, under nitrogen protection, reflux and stir for 24h. After the reaction was completed, it was extracted with dichloromethane, washed with saturated brine, dried, concentrated, and subjected to column chromatography to obtain 1.0 g of solid A-6 with a yield of 76%. Mass spectrum: theoretical value m / e, 486.2; measured value 486.1. 1H-NMR (500MHZ, CDCl 3 ,): 8.62(2H), 8.46(2H), 7.84(2H), 7.79-7.70(6H), 7.59(2H), 7.56-7.57(4H), 7.39(2H), 7.33(2H).
[0083]
Embodiment 3
[0084] Embodiment three: the preparation of compound B-2
[0085] a) N-(6-bromo-pyridin-2-yl)-δ-carboline (a9): using 2,6-dibromopyridine and δ-carboline as raw materials, the synthesis steps are the same as a3, mass spectrum: theoretical value m / e, 323.0, 325.0; found value 323.1, 325.1.
[0086] b) Compound B-2: using 3-(δ-carbolyl)-phenylboronic acid pinacol ester (a4) and N-(6-bromo-pyridin-2-yl)-α-carboline as raw materials, The synthesis method is the same as A-5. Mass spectrum: theoretical value m / e, 487.2; measured value 487.3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com