Pharmaceutical composition containing human parathyroid hormone administered through oral mucosa
A parathyroid hormone and oral mucosa technology, applied in the field of biomedicine, can solve the problems of undisclosed rhPTH, oral mucosa drug delivery system, slow progress, technical difficulties, etc., to achieve improved compliance, good clinical application prospects, no drug administration pain effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Pharmaceutical composition containing rhPTH(1-34)
[0045] Table 1. The ratio of each component of the pharmaceutical composition A-X
[0046]
[0047]
[0048]
[0049] Thaw the recombinantly prepared rhPTH(1-34) protein stock solution (purity 98%, concentration 2mg / ml) and rhPH20 protein stock solution (purity 98%, concentration 2mg / ml) respectively at room temperature or 2-8°C, and after thawing, Store at 2-8°C.
[0050] Accurately weigh the mucosal adsorbents according to the proportions of the ingredients of the pharmaceutical composition A-X in Table 1, dissolve them in water for injection and prepare a mixed solution, add the thawed rhPH20 protein stock solution, mix well, and finally add rhPTH (1-34) Stir and mix the protein stock solution, filter and sterilize with a filter membrane with a pore size of 0.22 μm under aseptic conditions to obtain pharmaceutical compositions A-X respectively.
[0051] According to the requirements of preparation product...
Embodiment 2
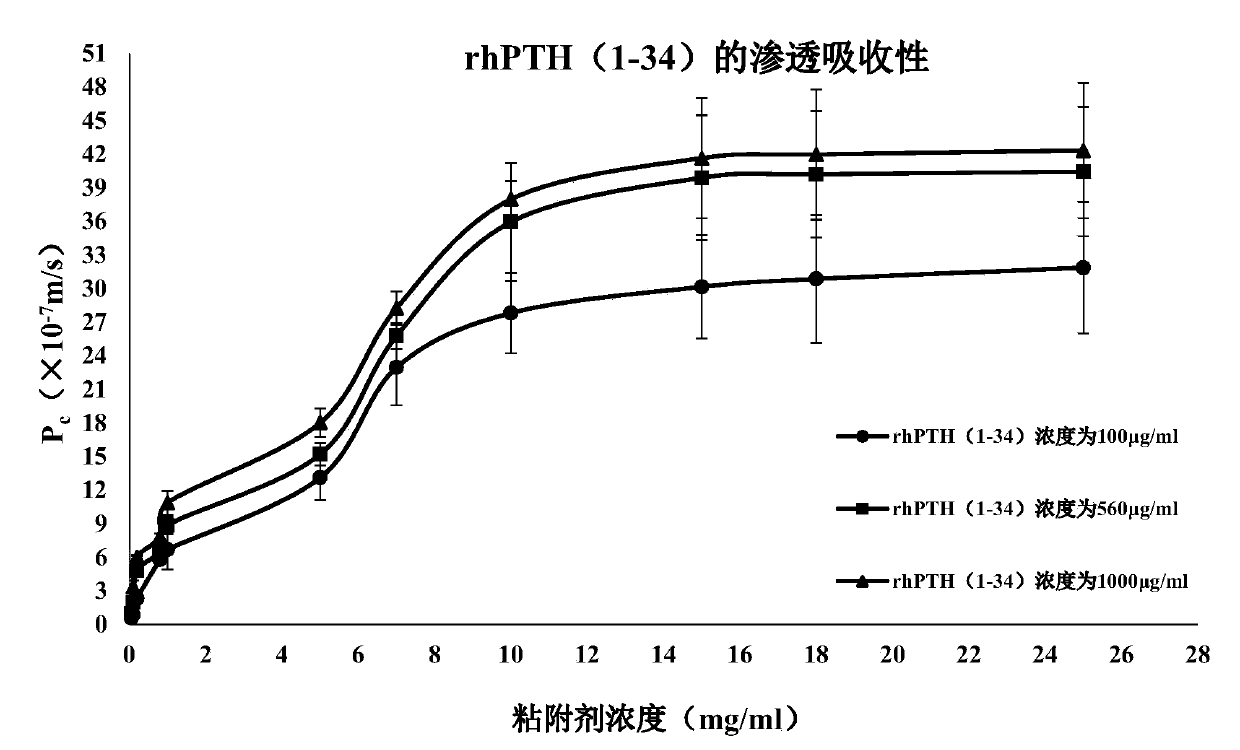
[0066] Example 2 Osmotic absorption detection
[0067] According to the literature report (Portero A.Pharm Res, 2002,19(2):169-174), TR146 esophageal squamous carcinoma cells (derived from ATCC) were used as oral mucosal epithelial cell permeation and transport model to detect Example 1 and comparative The osmotic absorbability of the pharmaceutical composition obtained in Examples 1-4.
[0068]Aspirate the DMEM culture solution in the upper and lower chambers of the insert culture dish, and wash the TR146 cells with HBSS buffer. Then add HBSS solution into the upper and lower chambers respectively, and after equilibrating for 1 hour, suck out the HBSS solution in the upper chamber, and add 2 mL of the test sample (pharmaceutical composition A-X, pharmaceutical composition C1-C4). Take a sample of 100 μL from the lower chamber at the set time, and immediately add an equal amount of HBSS solution. Using chromatographic method, sample injection analysis, record the obtained pe...
Embodiment 3
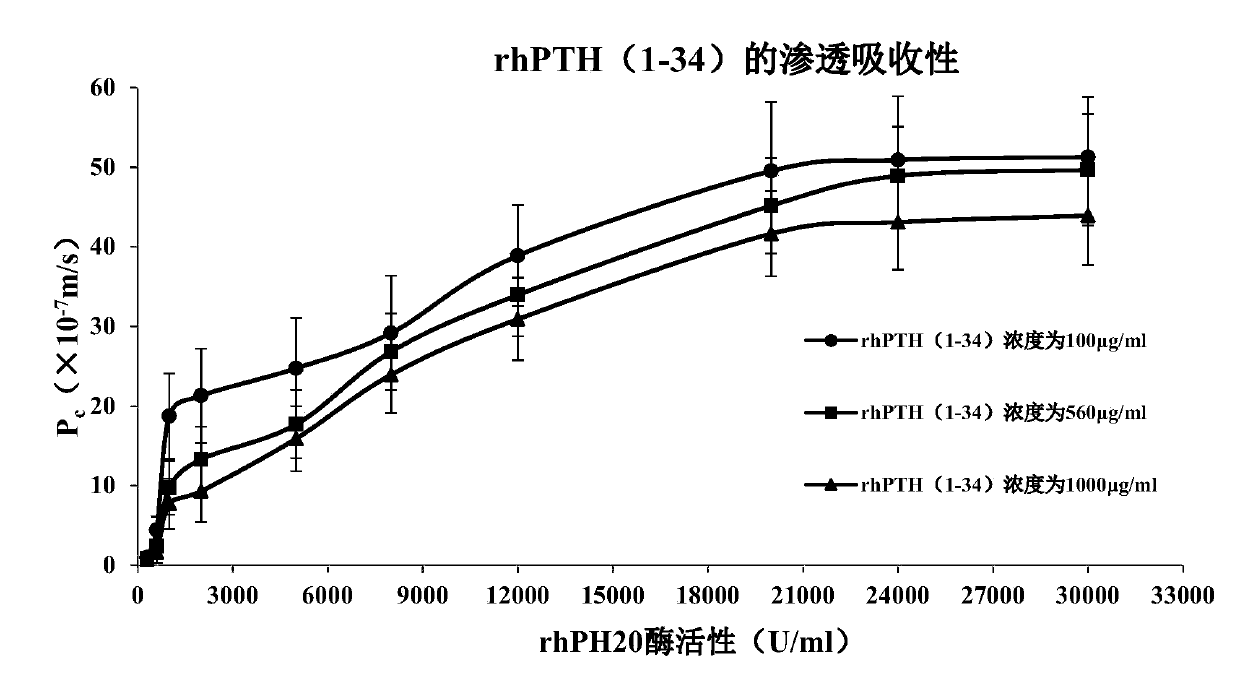
[0078] Example 3. Effects of different enzymatic activities on the penetration and absorption of rhPTH(1-34) through TR146 oral mucosal epithelial cells by the absorption penetration enhancer rhPH20
[0079] According to the composition ratio of D1-D10 in the following table 6.1, the enzyme activity of the absorption penetration enhancer rhPH20 is 300, 600, 1000, 2000, 5000, 8000, 12000, 20000 U / ml respectively, and the same method as in Example 1 is adopted Compositions containing rhPH20 with different enzymatic activities were prepared, and the effect of rhPH20 with different enzymatic activities on the osmotic absorption of rhPTH(1-34) through TR146 oral mucosal epithelial cells was detected using the same method as in Example 2.
[0080] The experimental results are shown in Table 6.2 and figure 1 As shown, the pharmaceutical composition D1-D10, when the rhPH20 enzyme activity is 1000~20000U / ml, can effectively promote the penetration and absorption of rhPTH (1-34) through...
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com