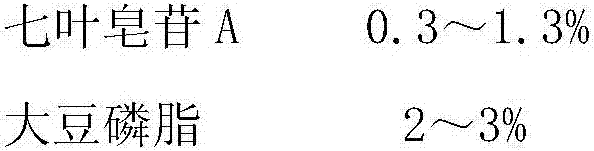Aescin A liposome gel and a preparing method thereof
A technology of liposome gel and aescin, which is applied in liposome delivery, liquid delivery, pharmaceutical formulations, etc., can solve the problems of poor transdermal absorption effect and large skin irritation, and achieve good transdermal absorption effect , reduce incidence, good anti-inflammatory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
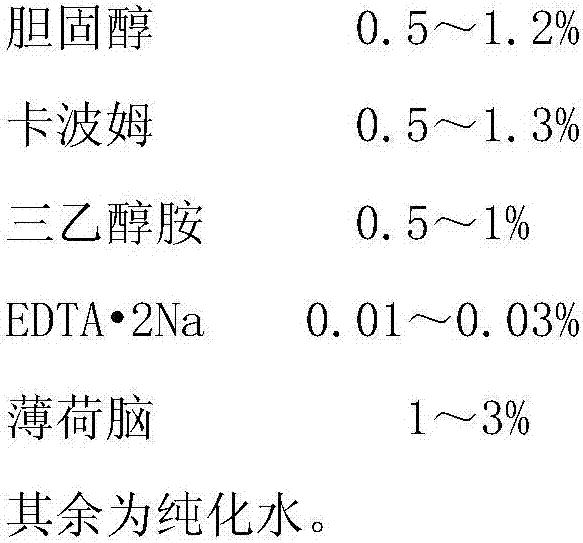
[0021] Raw material prescription:
[0022]
[0023] Preparation Process:
[0024] 1) Preparation of liposomes: Take 5g of aescin A, 20g of soybean lecithin and 5g of cholesterol, add 50g of absolute ethanol, stir at 60°C to dissolve, inject the resulting ethanol solution into the liposome at a speed of 15ml / min through a peristaltic pump 250g of purified water at a constant temperature of 60°C was stirred for 30 minutes to make a liposome suspension; then the liposome suspension was placed in a liposome extruder and passed through PC filters with a pore size of 200nm and 100nm in sequence. The membranes were extruded 4 times each to obtain 285 g of the extruded solution; finally, the method of membrane dialysis was used to dilute the extruded solution by adding 200 g of purified water, and then dialyzed 3 times to remove the ethanol in the extruded solution to obtain aescin A liposome 420g;
[0025] The encapsulation efficiency and particle size of the liposomes were dete...
Embodiment 2
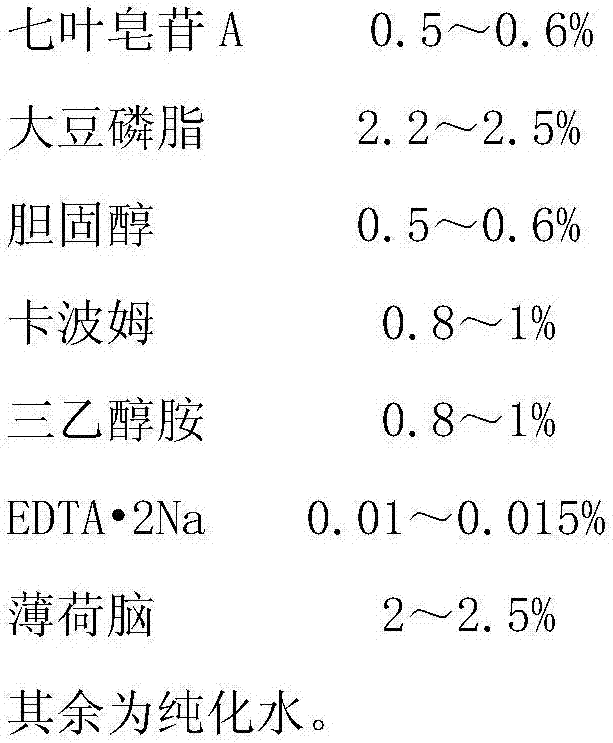
[0030] Raw material prescription:
[0031]
[0032] Preparation Process:
[0033] 1) Preparation of liposomes: Take 3g of aescin A, 25g of soybean lecithin and 10g of cholesterol, add 100g of absolute ethanol, stir at 60°C to dissolve, and inject the resulting ethanol solution into the liposome at a speed of 25ml / min through a peristaltic pump 300g of purified water at constant temperature to 60°C was stirred for 30 minutes to make a liposome suspension; then the liposome suspension was placed in a liposome extruder, and passed through PC filters with a pore size of 200nm and 100nm in sequence. Each membrane was extruded 4 times to obtain 350 g of extruded liquid; finally, membrane dialysis was used to dilute the extruded liquid with 300 g of purified water, and then dialyzed 3 times to remove ethanol in the extruded liquid to obtain aescin A liposome 490g;
[0034] The encapsulation efficiency and particle size of the liposomes were detected according to the literature m...
Embodiment 3
[0039] Raw material prescription:
[0040]
[0041] Preparation Process:
[0042] 1) Preparation of liposomes: Take 8g of aescin A, 15g of soybean lecithin and 4g of cholesterol, add 30g of absolute ethanol, stir at 60°C to dissolve, inject the obtained ethanol solution into the liposome at a speed of 5ml / min through a peristaltic pump 150g of purified water at constant temperature to 60°C was stirred for 30 minutes to make a liposome suspension; then the liposome suspension was placed in a liposome extruder and passed through PC filters with a pore size of 200nm and 100nm in sequence. The membranes were extruded 4 times each to obtain 175 g of the extruded solution; finally, the method of membrane dialysis was used to dilute the extruded solution with 150 g of purified water, and then dialyzed 3 times to remove the ethanol in the extruded solution to obtain aescin A liposome 290g;
[0043] The encapsulation efficiency and particle size of the liposomes were detected acco...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com