A kind of method for high-selectivity oxidation of benzylamine green synthesis of n-benzyl butylamine
A technology of benzylamine oxide and synthesis method, which is applied in chemical instruments and methods, imino compound preparation, metal/metal oxide/metal hydroxide catalyst, etc., and can solve high reaction temperature, weak visible light response, and photocatalytic activity Low-level problems, to achieve the effect of low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The present invention prepares the photocatalyst with the different molar ratios of chromium tungstate and bismuth tungstate in the cadmium tungstate-bismuth tungstate composite photocatalyst:
[0023] Preparation of cadmium tungstate: Dissolve 1.234g cadmium nitrate in 40ml deionized water and mix with 0.240g ethylenediamine solution, stir well to form solution A; dissolve sodium tungstate in 40ml water to obtain solution B; mix and stir AB for 10min , to obtain solution C; then the obtained solution C was crystallized at 180° C. for 20 hours. The crystallization solution is separated by cooling and filtration, washed three times with deionized water and once with absolute ethanol, and dried at 80°C for 6 hours to obtain cadmium tungstate;
[0024] Preparation of composite material: Dissolve 0.041g of sodium tungstate in 10ml of ethylene glycol solution, stir well to form solution D; dissolve 0.121g of bismuth nitrate in 10ml of ethylene glycol solution, stir well to f...
Embodiment 2~5
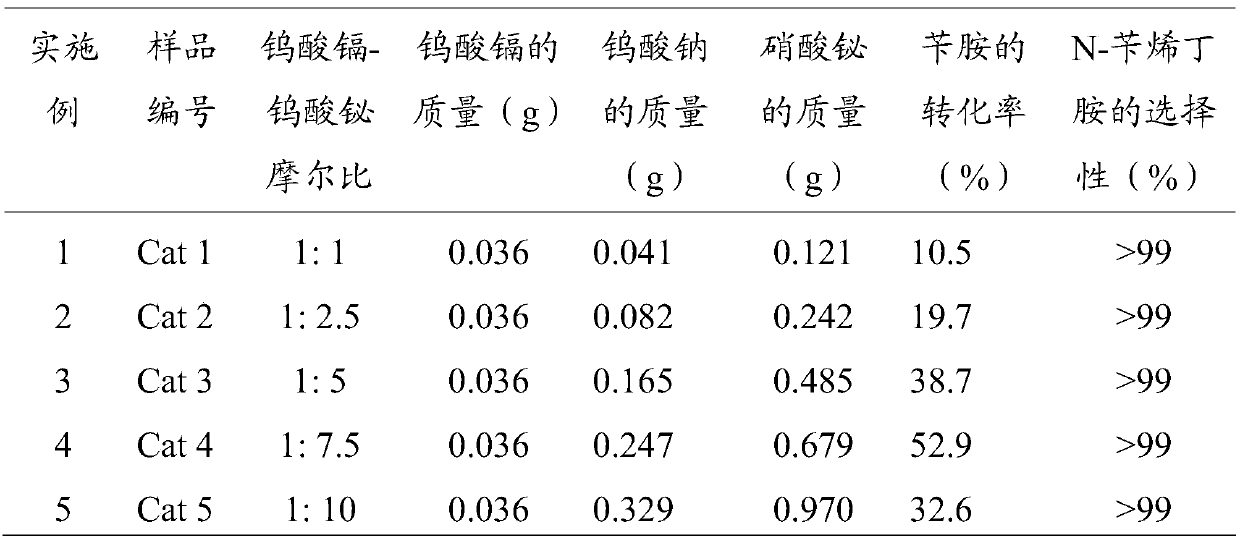
[0028] For cadmium tungstate and bismuth tungstate composite photocatalysts with different molar ratios, the operation steps are similar to those in Example 1, only the amount of sodium tungstate and bismuth nitrate in the composite material is changed, and the rest of the conditions are unchanged, and the sample number is Cat 2, Cat 3, Cat 4, Cat 5. See Table 1 for the composite catalyst conditions and reaction results prepared in Examples 1-5.
[0029] Table 1 Preparation conditions and reaction results of composite photocatalysts with different molar ratios
[0030]
[0031] It is found from Table 1 that the ratio of Cat 4 cadmium tungstate-bismuth tungstate makes the conversion rate of benzylamine 38.7%, the selectivity of N-benzylbutylamine is greater than 99%, and has the best photocatalytic effect.
Embodiment 6~10
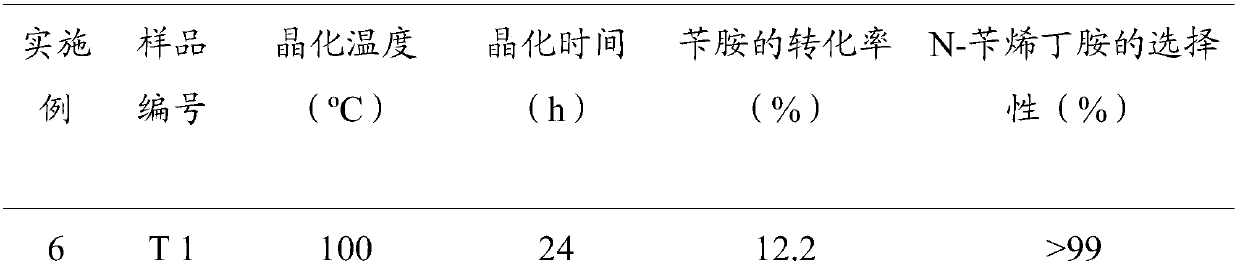
[0033] According to the step 4 of the embodiment in which the molar ratio of chromium tungstate and bismuth tungstate in the reaction mixture has the best effect is 1:7.5, the remaining conditions remain unchanged, and the crystallization temperature prepared by the composite photocatalyst is changed, respectively using 100°C, 120°C, After crystallization at 140°C, 180°C, and 200°C for 24 hours, the samples were respectively coded as T 1, T 2, T 3, T 4, and T 5, and the rest of the operation steps were similar to those in Example 4. See Table 2 for the composite catalyst conditions and reaction results prepared in Examples 6-10.
[0034] Table 2 The molar ratio of chromium tungstate and bismuth tungstate is 1:7.5 and different crystallization temperature conditions and reaction results
[0035]
[0036]
[0037] It is found from Table 2 that different benzylamine conversion rates are obtained at different crystallization temperatures, and the temperature is compared with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com