Method for high-purity and high-yield preparation of p-chlorophenylboronic acid
A high-yield technology of p-chlorophenylboronic acid, applied in the fields of medicinal chemistry and material chemistry, can solve the problems of large environmental pollution, low purity, and many solid wastes, and achieves reduced environmental pollution, high product purity, and good reaction effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A method for preparing p-chlorophenylboronic acid with high purity and high yield is provided, and the specific preparation process of said p-chlorophenylboronic acid is:
[0024] (1) Grignard reagent preparation:
[0025] Under the protection of nitrogen, add 666.46g of newly produced magnesium chips and 13L of butyl ether into the 50L reaction kettle, stir, and heat to 120°C. Add a small amount of p-chlorophenylmagnesium bromide butyl ether solution to the reaction system, and after stirring for 5 minutes, add dropwise a mixture of 3.84kg of 1,4-dichlorobenzene and 13L of n-butyl ether, trigger for 10min, and complete the dropwise addition after 2h. Maintain stirring for 8h, stop the reaction, and obtain the Grignard reagent.
[0026] (2) Preparation of p-chlorophenylboronic acid:
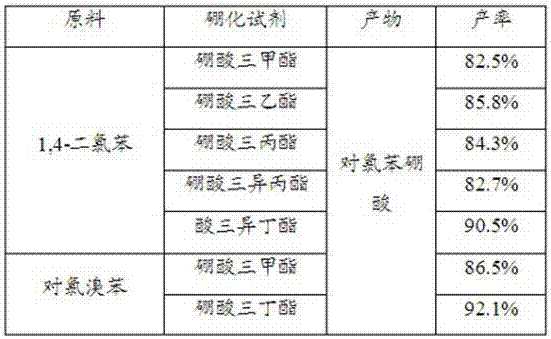
[0027] Under nitrogen protection, tributyl borate (7.21kg, 31.33mol) and 10L butyl ether were added to a 100L low-temperature reactor, and the temperature was lowered to -70°C under mech...
Embodiment 2
[0032] The difference between this embodiment and the specific implementation case 1 is that p-chlorobromobenzene was added dropwise in step (1), and 3.76 kg of the product was obtained, with a yield of 92.1% and an HPLC content of 99.5%. Other implementation manners are the same as the specific case 1.
Embodiment 3
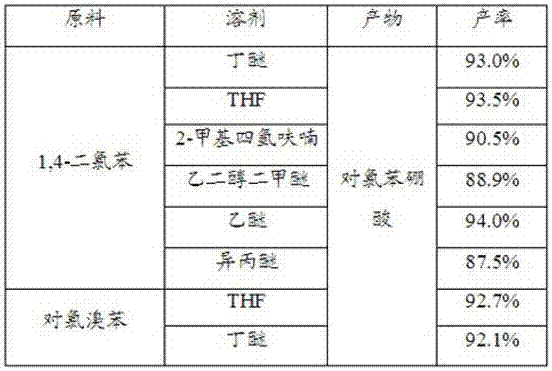
[0034] The difference between this embodiment and specific implementation cases 1 to 2 is that the solvents used in the reaction of step (1) and step (2) are diethyl ether, ethylene glycol dimethyl ether, isopropyl ether, THF, and 2-methyltetrahydrofuran. Other implementation modes are the same as the specific implementation cases 1 to 2. The reaction results are shown in the table below.
[0035]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com