Hydrodechlorination method for high chlorine acetone
A technology for hydrodechlorination and high-chlorinated acetone is applied in the field of environmental pollution control and resource utilization, which can solve the problems of complex process, harsh conditions, explosion, etc., and achieves overcoming complex process, mild processing conditions and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
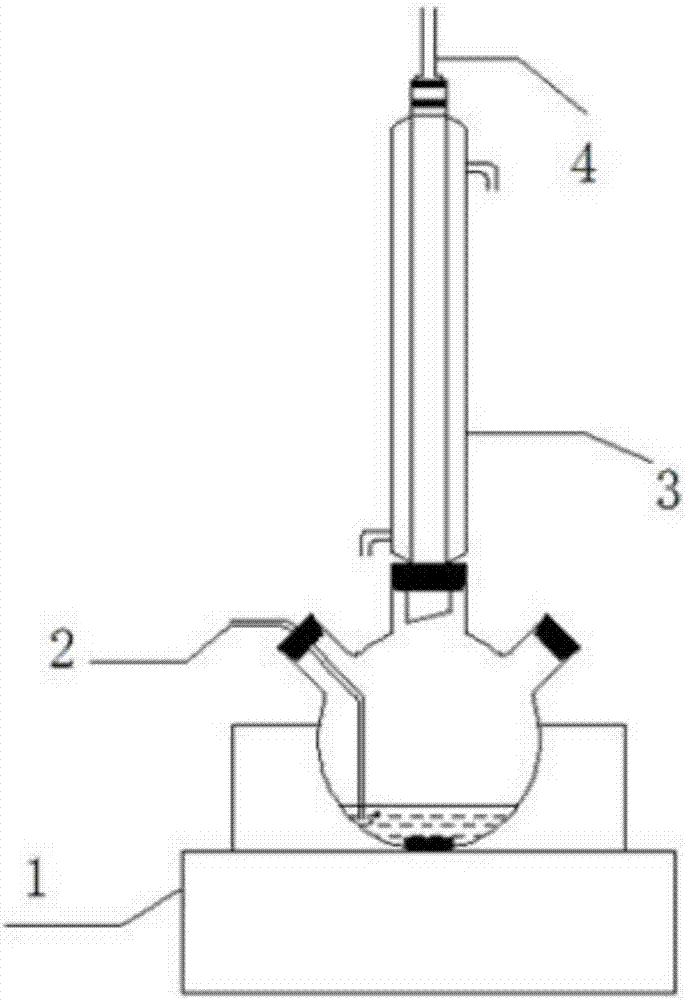
[0030] Get 10mL of perchloracetone residue on a certain folic acid production line in a three-necked flask, add 100mL deionized water to mix, add 0.5g carbon-based hydrogenation catalyst and 0.25g NaOH solid, magnetically stir, and hydrogen (10%H 2 ) into the system, the flow rate is 50mL / min, the reaction temperature is 80-100°C, the reaction is continuous for 6-12 hours, the residue completely disappears, and the water phase product is yellow.
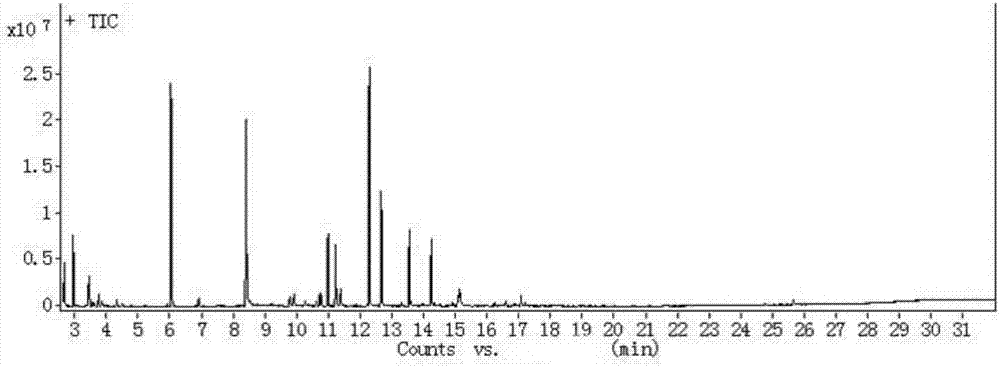
[0031] The product was separated from the carbon-based hydrogenation catalyst, and the aqueous phase was added dropwise with AgNO 3 The solution produces a white precipitate, which proves that there is Cl - produce. After the high chloroacetone residue was extracted with methyl tert-butyl ether, it was monitored by gas chromatography-mass spectrometry and found to contain 1,1,3-trichloroacetone, 1,1,1-trichloroacetone, 1,1,3 ,3-tetrachloroacetone, 1,1,1,3-tetrachloroacetone, pentachloroacetone and hexachloroacetone. Among them, th...
Embodiment 2
[0035] The difference between this embodiment and Example 1 is that the amount of NaOH solid added is 0.5 g, and other reagents added, reaction temperature, and reaction time are the same. After the reaction, the residue disappeared completely, and the aqueous phase product was yellow, and darker than the product obtained in Example 1.
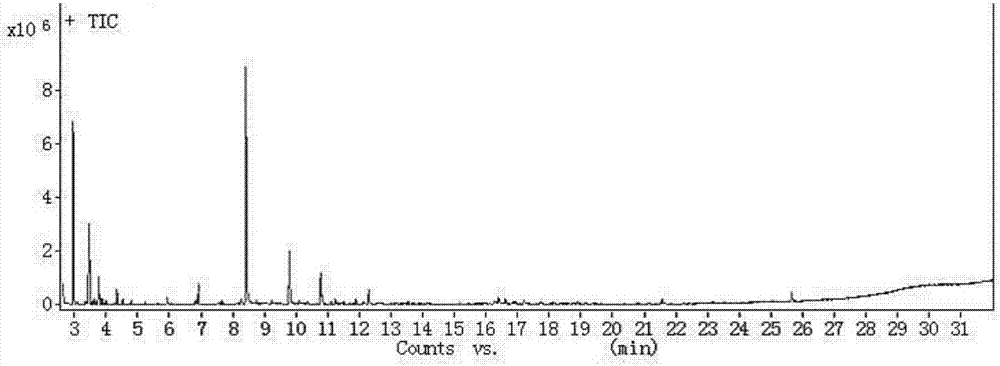
[0036] The product was separated from the carbon-based hydrogenation catalyst, and the aqueous phase was added dropwise with AgNO 3 The solution produces a white precipitate, which proves that there is Cl - produce. After the water phase is extracted with methyl tert-butyl ether, it is monitored by gas chromatography-mass spectrometry. The monitored chloroacetone substances are 1,1,3-trichloroacetone, a small amount of 1,1,3,3-tetrachloroacetone With a very small amount of 1,3-dichloroacetone, the overall reaction of the dechlorination reaction that takes place is the same as in Example 1.
[0037] The content of 1,1,3-trichloroacetone in t...
Embodiment 3
[0039] The difference between this example and Example 1 is that the amount of NaOH solid added is 1.0 g, and other reagents added, reaction temperature, and reaction time are the same. After the reaction, the residue disappeared completely, and the product in the aqueous phase was yellow, and darker than the products obtained in Example 1 and Example 2.
[0040] The product was separated from the carbon-based hydrogenation catalyst, and the aqueous phase was added dropwise with AgNO 3 The solution produces a white precipitate, which proves that there is Cl - produce. After the aqueous phase is extracted with methyl tert-butyl ether, it is monitored by gas chromatography-mass spectrometry. Among the monitored chloroacetone substances are 1,1,3-trichloroacetone, a small amount of 1,1,3,3-tetrachloro Acetone and very small amounts of 1,3-dichloroacetone.
[0041] The content of 1,1,3-trichloroacetone in the product after dechlorination of high-chlorine acetone residue is betw...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com