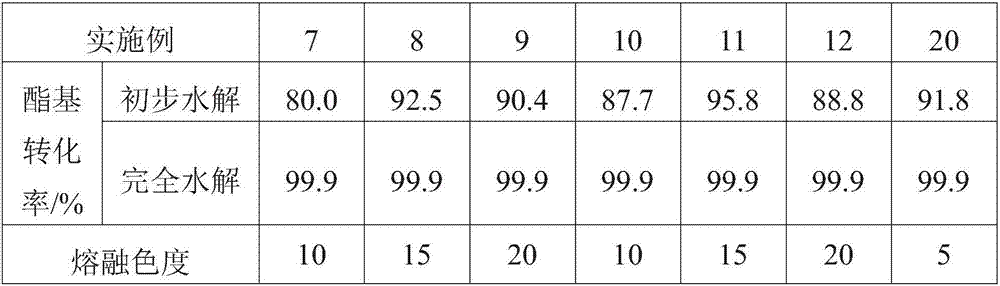
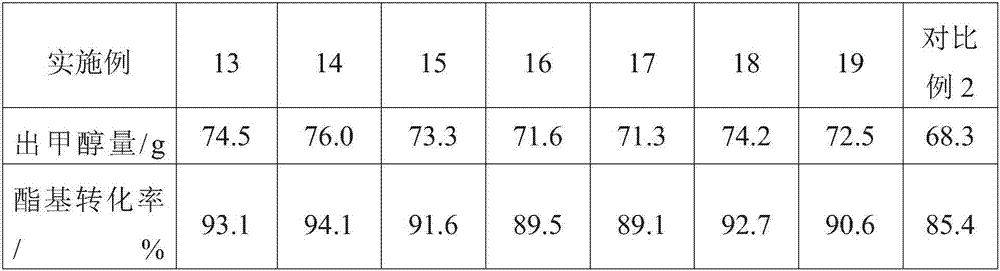
Method for purifying C4 to C6 dibasic acid monomers from adipic acid byproduct mixed dibasic acid
A technology for mixing dibasic acid and dibasic acid dimethyl ester, which is applied in the field of purifying C4-C6 dibasic acid monomers, can solve the problems of high separation and purification operation cost, incomplete hydrolysis reaction, consumption of a large amount of magnesium oxide, etc. The effect of improving reaction efficiency and saving reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Add 2000g of DBA and 2000g of methanol melted at 120°C to a 5L reaction kettle, add 1.23g of concentrated nitric acid, and perform esterification at 65°C for 24h.
[0056] Stand to separate layers, take the supernatant and distill:
[0057] First distill methanol and water: gradually heat up to 100°C under normal pressure, then gradually reduce the pressure to 30KPa, distill until no liquid comes out of the top of the tower, and distill 1630g of methanol and water;
[0058] Afterwards, the pressure was gradually reduced to 1KPa, and the distillate at the top temperature of 60-95°C was collected to obtain 1830g of dibasic acid dimethyl ester mixture, the main components of which were as follows:
[0059] Dimethyl succinate 23.98wt%,
[0060] Dimethyl glutarate 62.25wt%,
[0061] Dimethyl adipate 13.77wt%.
[0062] 420g remained at the bottom of the kettle.
Embodiment 2
[0064] 2285g of DBA slices and 1715g of methanol were added to a 5L reaction kettle, 6.15g of concentrated nitric acid was added, and esterified at 90°C for 16h.
[0065] Stand to separate layers, take the supernatant and distill:
[0066] First distill methanol and water: firstly under normal pressure, gradually raise the temperature to 100°C, then gradually reduce the pressure to 30KPa, distill until no liquid comes out from the top of the tower, distill out 1280g of methanol and water.
[0067] Afterwards, the pressure was gradually reduced to 5KPa, and the fraction at the top temperature of 85-125°C was collected to obtain 2050g of dibasic acid dimethyl ester mixture, the main components of which were as follows:
[0068] Dimethyl succinate 24.30wt%,
[0069] Dimethyl glutarate 60.05wt%,
[0070] Dimethyl adipate 15.65 wt%.
[0071] 600g remained at the bottom of the kettle.
Embodiment 3
[0073]Add 1600g of DBA slices and 6400g of methanol into a 10L reaction kettle, add 24.6g of concentrated nitric acid, and esterify at 72°C for 8h.
[0074] Stand to separate layers, take the supernatant and distill:
[0075] First distill methanol and water: firstly under normal pressure, gradually raise the temperature to 100°C, then gradually reduce the pressure to 30KPa, distill until no liquid comes out from the top of the tower, distill out 6050g of methanol and water.
[0076] Afterwards, the pressure was gradually reduced to 10KPa, and the fraction at the top temperature of 100-145°C was collected to obtain 1500g of dibasic acid dimethyl ester mixture, the main components of which were as follows:
[0077] Dimethyl succinate 24.30wt%,
[0078] Dimethyl glutarate 62.98wt%,
[0079] Dimethyl adipate 12.72wt%.
[0080] 290g remained at the bottom of the kettle.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com