Photochemical demethylation method for N6-methyladenine
A methyladenine and photochemical technology, applied in the fields of chemical biology and organic chemistry, can solve the problems of low conversion efficiency, excess, low biological tolerance, etc., and achieve the effect of wide selection range, precise and controllable adjustment advantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] with vitamin B 2 N for photosensitizer under 470nm wavelength blue light and Selectfluor 6 -Methyladenine demethylation: add N to the reaction tube in turn 6 - Methyladenine (0.2mmol, 29.8mg), Selectfluor (0.44mmol, 155.8mg), Vitamin B 2 (0.02mmol, 7.5mg), add 4ml H under the protection of nitrogen 2 O / CH 3 CN(1 / 1) solution, irradiated under blue light with a wavelength of 470nm, stirred for 3 hours, added an appropriate amount of sodium bicarbonate until the pH of the reaction solution was 7, spin-dried the liquid, and column chromatography (n-BuOH / H 2 O / MeOH=4 / 1 / 1) isolated 19 mg of adenine with a yield of 71%. 1 H NMR (DMSO-d 6 , 300MHz) δ12.8(s, 1H), 8.1(d, J=5.4Hz, 2H), 7.1(s, 2H).
Embodiment 2
[0040] with vitamin B 2 N for photosensitizer under 470nm wavelength blue light and Selectfluor 6 - Demethylation of methyladenine deoxyribonucleoside: add N to the reaction tube in turn 6 - Methyladenine deoxyribonucleoside (0.1mmol, 26.5mg), Selectfluor (0.22mmol, 78mg), Vitamin B 2 (0.01mmol, 3.8mg), under nitrogen protection, add 2ml H 2 O / CH 3 CN(1 / 1) solution, irradiated under blue light with a wavelength of 470nm, stirred for 3 hours, added an appropriate amount of sodium bicarbonate until the pH of the reaction solution was 7, spin-dried the liquid, and column chromatography (CH 2 Cl 2 / MeOH=12 / 1) to isolate 15 mg of adenine deoxyribonucleoside with a yield of 60%. 1 H NMR (DMSO-d 6 ,300MHz)δ8.33(s,1H),8.13(s,1H),7.31(s,2H),6.36-6.31(m,1H),5.31(d,J=4.2Hz,1H),5.26-5.22 (m, 1H), 4.42-4.38(m, 1H), 3.89-3.86(m, 1H), 3.65-3.60(m, 1H), 3.58-3.48(m, 1H).
Embodiment 3
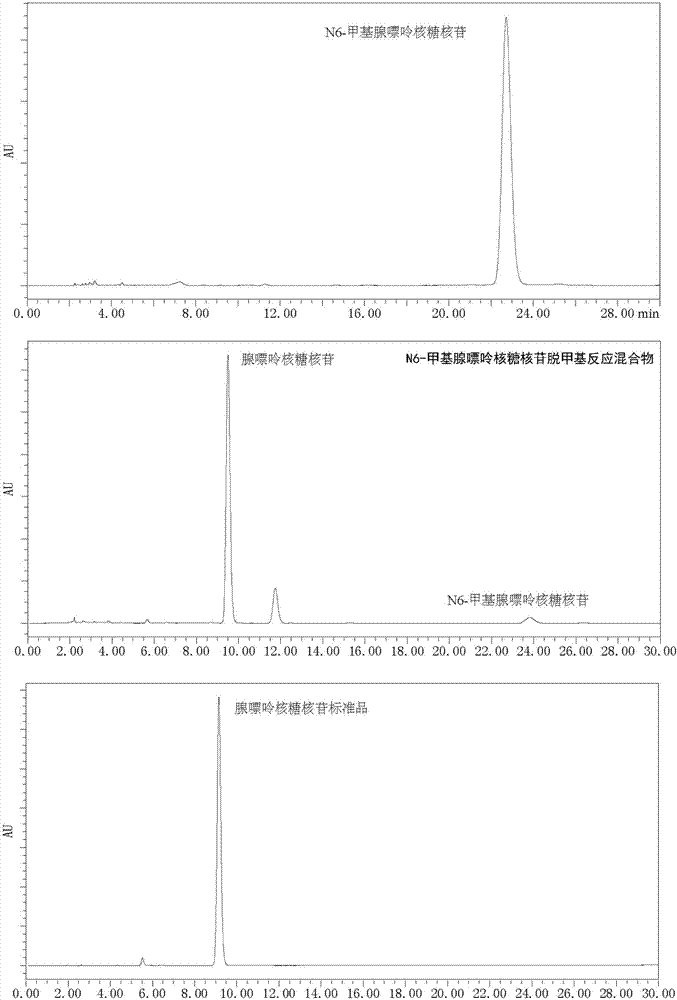
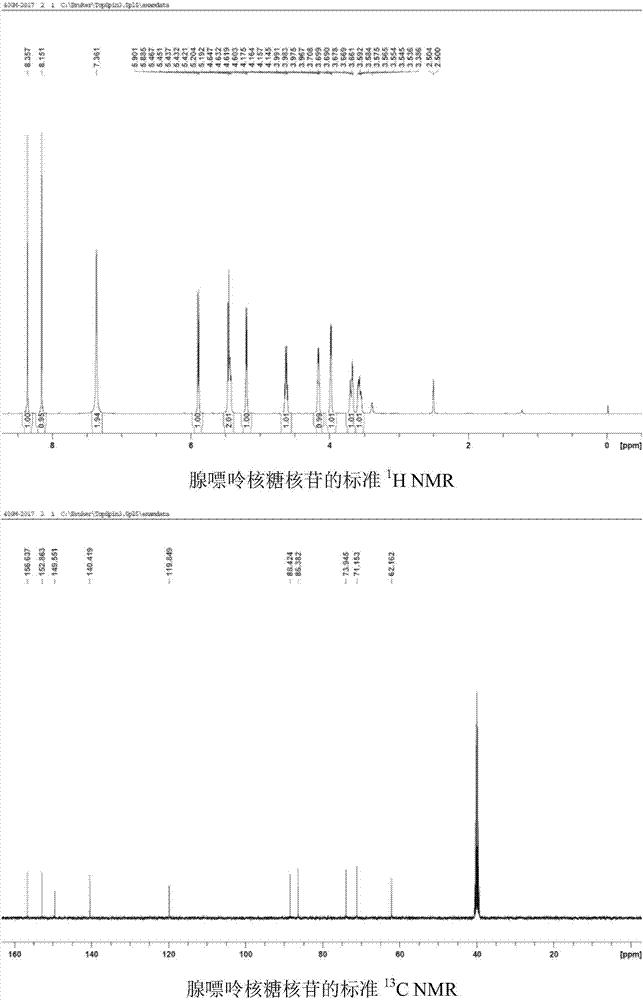
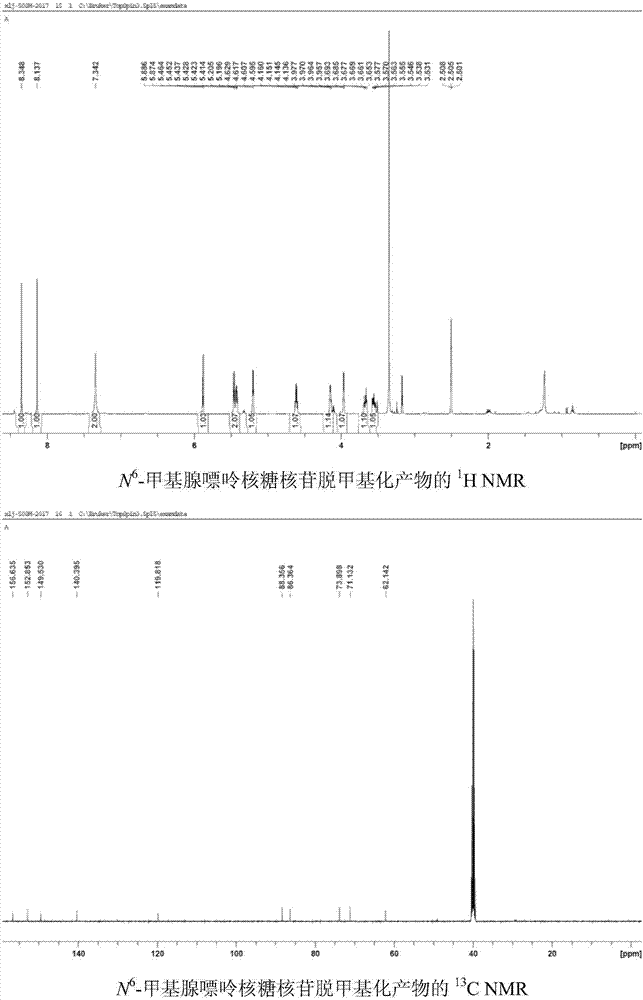
[0042] Use 1,4-dicyanobenzene as photosensitizer to make N 6 - Demethylation of methyl adenine ribonucleoside: add N to the reaction tube in turn 6 -Methyl adenine ribonucleoside (0.1mmol, 28.1mg), Selectfluor (0.22mmol, 77.9mg), 1,4-dicyanobenzene (0.01mmol, 1.3mg), add 2ml of H under nitrogen protection 2 O / CH 3 CN(1 / 1) solution, irradiated under 303nm wavelength ultraviolet rays, stirred for 8 hours, added an appropriate amount of sodium bicarbonate until the pH of the reaction solution was 7, spin-dried the reaction solution, and column chromatography (CH 2 Cl 2 / MeOH=12 / 1) to isolate 25 mg of adenine ribonucleoside with a yield of 95%. 1 HNMR (DMSO-d 6 ,500MHz)δ8.35(s,1H),8.14(s,1H),7.34(s,2H),5.88(d,J=6.0Hz,1H),5.46-5.41(m,2H),5.20(d ,J=4.5Hz,1H),4.63-4.60(m,1H),4.16-4.14(m,1H),3.98-3.96(m,1H),3.69-3.65(m,1H),3.58-3.53(m ,1H). 13 C NMR (DMSO-d 6 ,125MHz)δ156.6,152.8,149.5,140.4,119.8,88.4,86.3,73.9,71.1,62.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com