Injection containing triamcinolone acetonide and PLGA sustained release microspheres, preparation method of injection and application of injection to preparation of medicine for treating osteoarthritis pain
A technology of slow-release microspheres and injections, applied in drug combination, drug delivery, antipyretics, etc., can solve the problems of patient burden, achieve the effects of reducing patient burden, prolonging analgesic time, and reducing the number of injections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
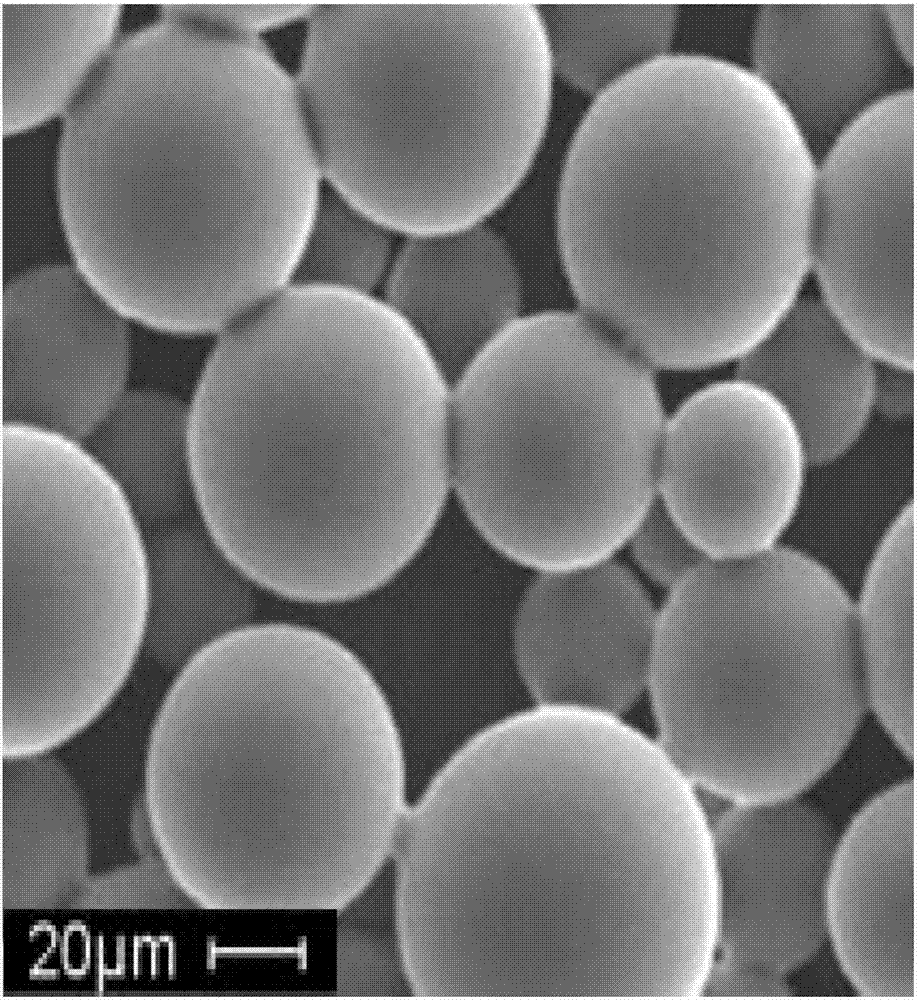
[0025] 1. Preparation of drug-loaded sustained-release microspheres
[0026] Composite PLGA sustained-release microspheres loaded with triamcinolone acetonide were prepared by solvent evaporation method. Proceed as follows:
[0027] Accurately weigh 50 mg of PLGA, dissolve it in 1 ml of dichloromethane, add 7.5 mg of triamcinolone acetonide, and hyaluronic acid freeze-induced layered vitrified particles (containing 5 mg of hyaluronic acid), vortex to fully dissolve, and form an organic phase. Draw the organic phase with a 1ml syringe, slowly inject it into 50ml of 3% PVA aqueous solution, stir with electric force at 1000rpm for 3 minutes, and continue magnetic stirring for 5 hours. The solidified drug-loaded microspheres were suction-filtered through a 0.22 μm microporous membrane, washed repeatedly with 500 ml of distilled water, collected the drug-loaded microspheres, and vacuum freeze-dried to obtain triamcinolone-loaded composite PLGA microspheres.
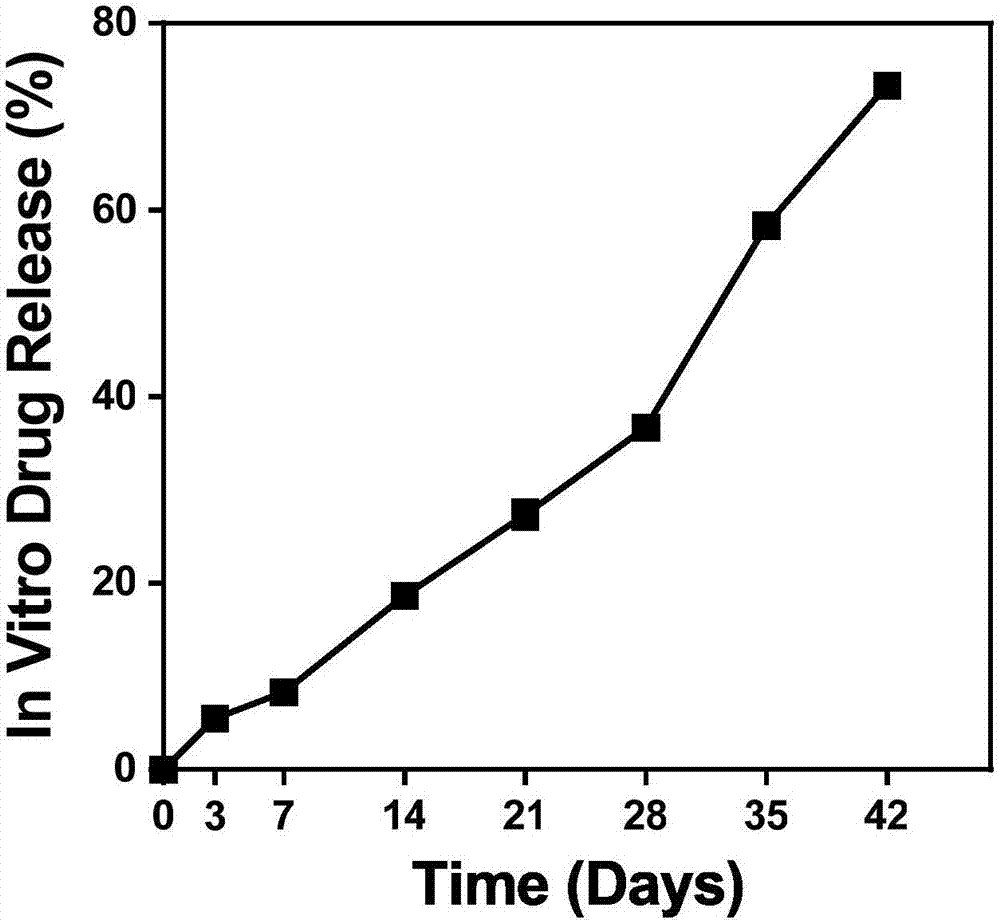
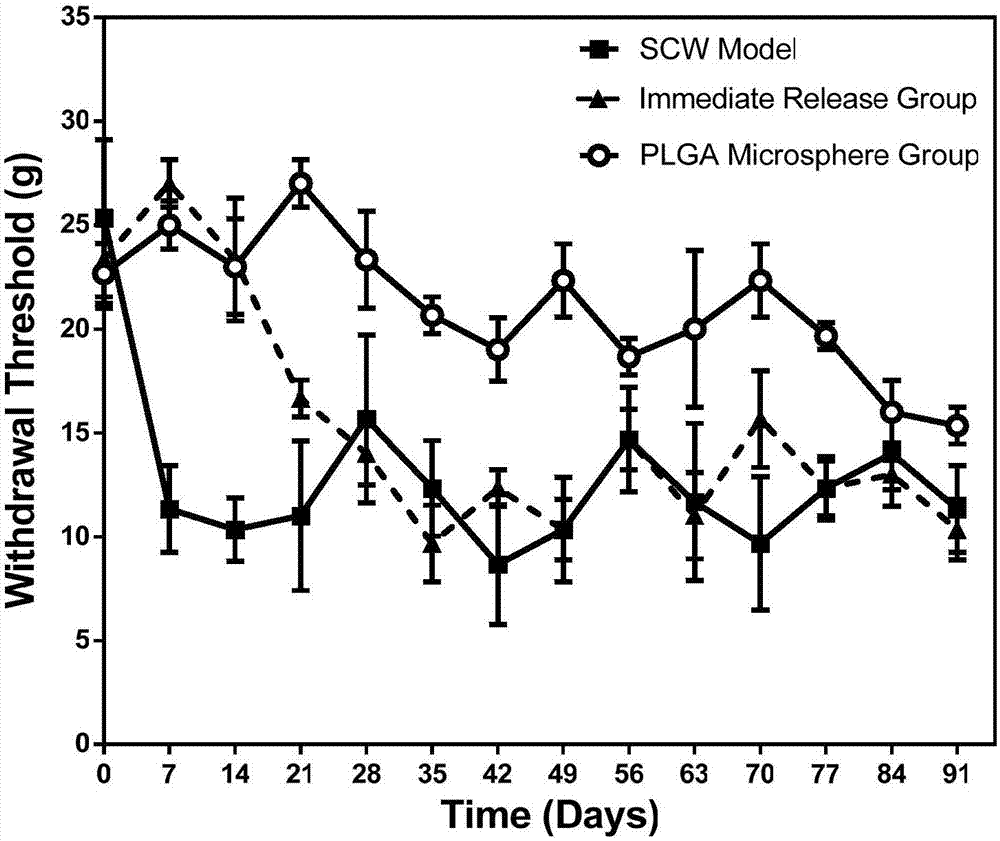
[0028] The experimen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com