Cancer immunotherapy using virus particles
A technology of virus particles and virus-like particles, applied in the direction of viruses, viral peptides, viruses/phages, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: In situ vaccination with plant-derived viroid nanoparticle immunotherapy inhibits metastatic cancer
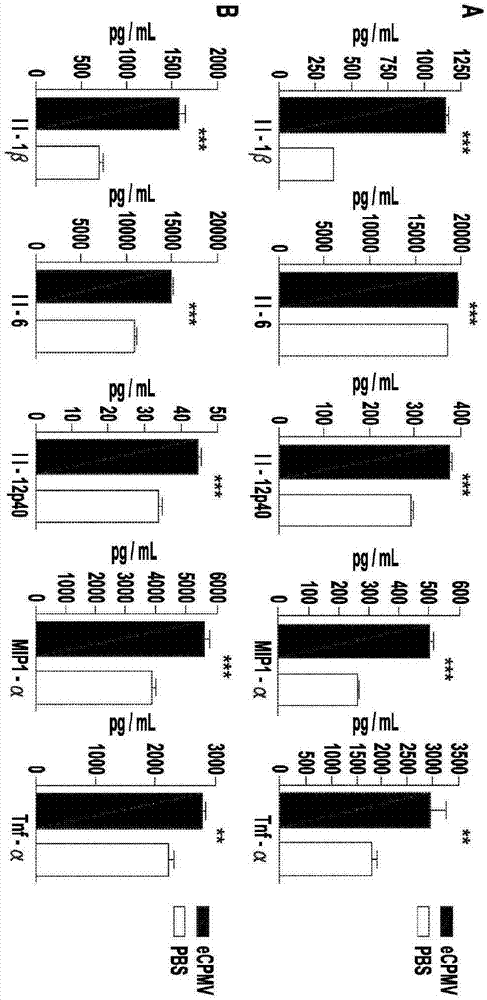
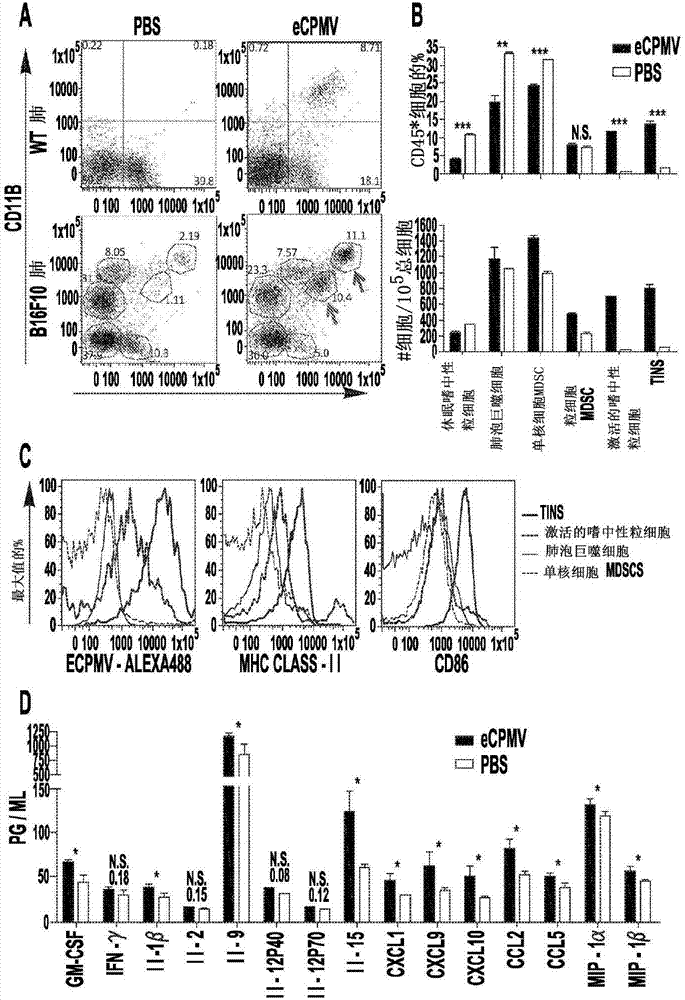
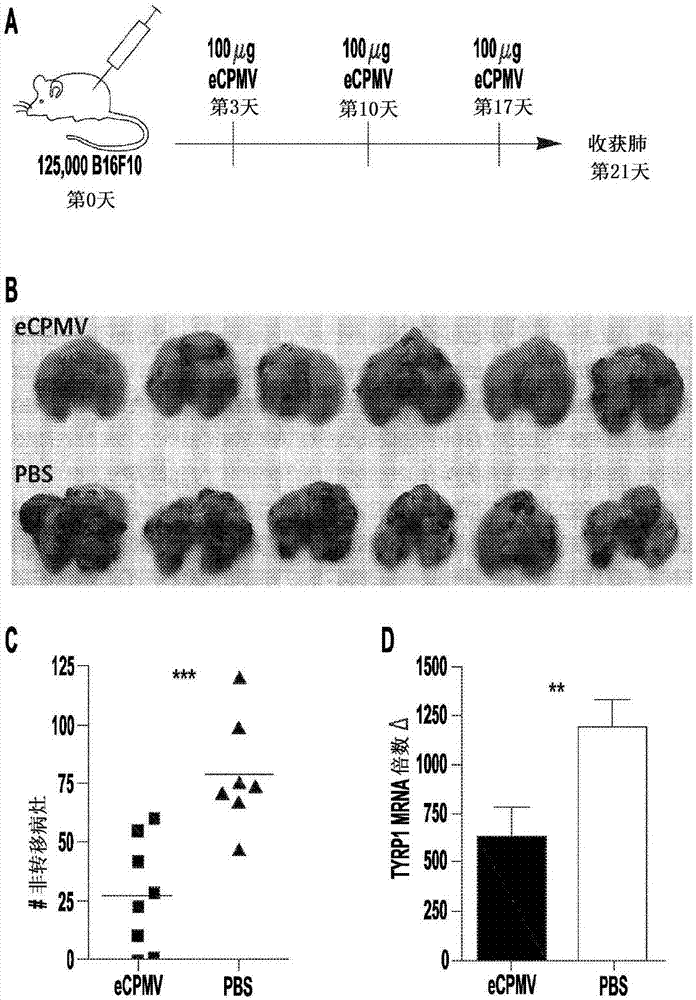
[0060] Current therapies are often ineffective against metastatic cancer, and emerging immunotherapies, while promising, are in the early stages of development. In situ vaccination refers to a process in which an immunostimulatory agent applied directly to the tumor alters the immunosuppressive microenvironment so that the immune system can respond efficiently to the tumor. The inventors hypothesized that treatment of lung tumor-bearing mice with viroid nanoparticles could modulate the immune environment of the lung and prevent metastatic injury of the B 16F10 class. This example shows that inhalation of self-assembled virus-like particles derived from cowpea mosaic virus (CPMV) inhibits tumor development in the lungs of mice following intravenous challenge. The inconsistency in tumor burden between CPMV- and PBS-treated mice was prominent and due to the p...
Embodiment 2
[0105] Example 2: eCPMV Treatment of Skin B16F10 Induces Systemic Anti-Tumor Immunity
[0106] like Image 6 As shown, the inventors established skin melanoma tumors, and injected eCPMV particles directly into the skin (100 μg / injection, arrows indicate injection days). The above treatment resulted in the complete disappearance of tumors in half of the mice. In cured mice, we then waited 4 weeks and challenged again with the same tumor cells, but we injected tumor cells on the opposite flank. The majority of these mice did not develop secondary tumors. For clarity, primary tumors were injected directly with particles, while mice bearing secondary tumors were left untreated. They must rely on a separate systemic antitumor immune memory. eCPMV was applied locally in the primary tumor, but systemic immunity was induced. "Re-challenged" mice that previously had B 16F10 skin tumors were injected directly, and shrunk and disappeared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com