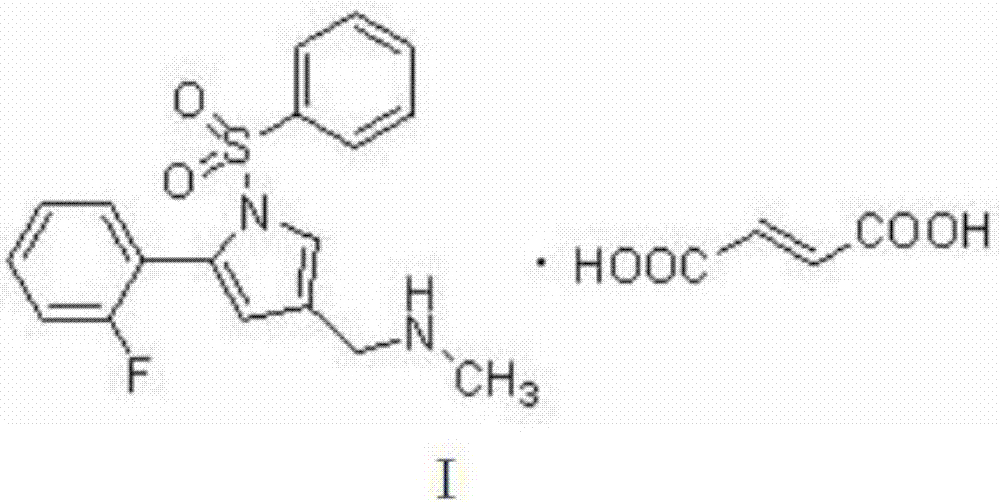
Pharmaceutical compound for treating peptic ulcer and preparation method thereof
A technology for pharmaceutical compounds and peptic ulcers, applied in the field of medicine, can solve problems such as the lack of purification methods for vonoprazan fumarate, difficulty in balancing yield and purity, and difficulty in hydrate preparation. It is suitable for large-scale production, The preparation method is simple and easy to operate, and the effect of improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
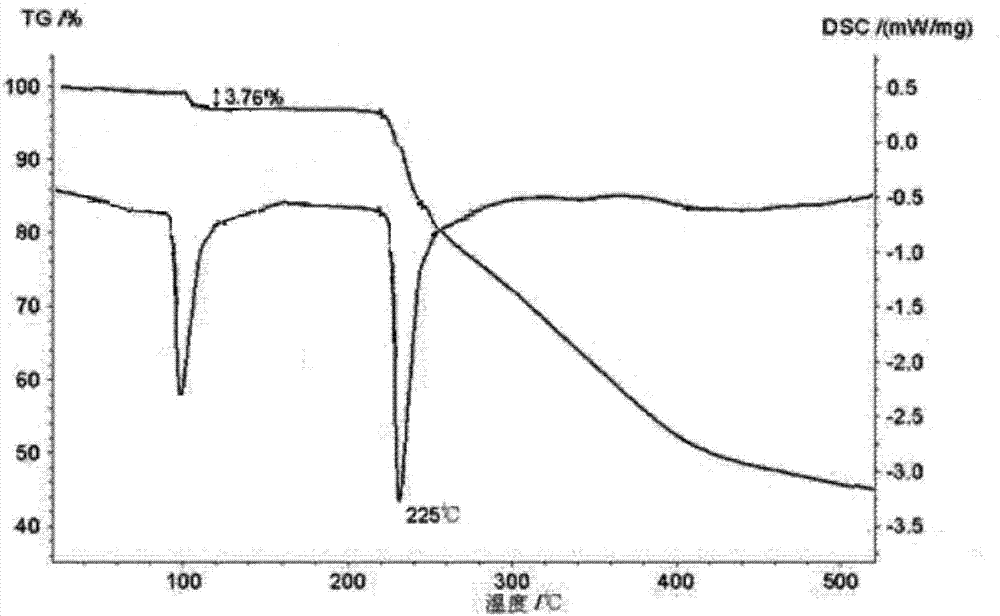
[0057] Embodiment 1: Preparation of fumaric acid Vonola monohydrate
[0058] (1) Take 100g of vonoprazan fumaric acid crude product, add tetramethylethylenediamine / water (volume ratio: 25:1.0) mixed solution (700ml), stir (40 rpm) to dissolve for 1-2min, Activated carbon decolorization, suction filtration;
[0059] (2) Reduce the filtrate of step (1) to -2°C, add dropwise (1.0mL / min) 700ml of pre-cooled tetramethylethylenediamine, and continue to cool down (3°C per 10 minutes) to -10°C Crystallization;
[0060] (3) Insulate and stir until the crystallization is complete, grow the crystal for 2 hours, filter with suction, wash with water, and dry at 40°C to obtain a white crystalline powder.
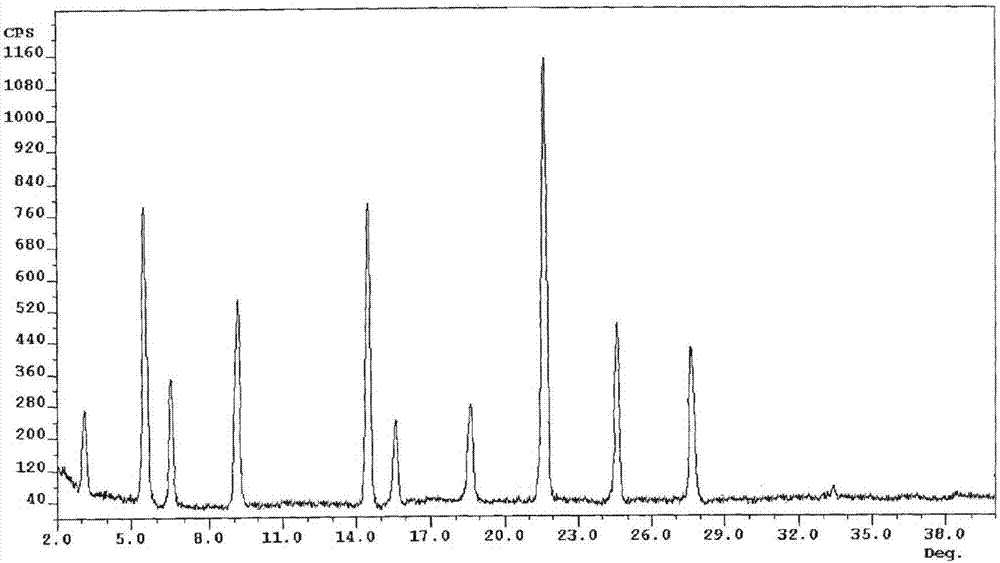
[0061] The X-ray powder diffraction spectrogram that the prepared white crystalline powder uses Cu-Kα ray measurement to obtain is shown in figure 1 .
Embodiment 2
[0062] Embodiment 2: Preparation of fumaric acid Vonola monohydrate
[0063] (1) Take 100g of vonoprazan fumaric acid crude product, add tetramethylethylenediamine / water (volume ratio: 25:1.2) mixed solution (500ml), stir (50 rpm) to dissolve for 1-2min, Activated carbon decolorization, suction filtration;
[0064] (2) Reduce the filtrate of step (1) to -2°C, add dropwise (1.5mL / min) 250ml of pre-cooled tetramethylethylenediamine, and continue to cool down (the cooling rate is 2°C per 10 minutes) to -9°C Crystallization;
[0065] (3) Insulate and stir until the crystallization is complete, grow the crystal for 3 hours, filter with suction, wash with water, and dry at 50°C to obtain a white crystalline powder.
[0066] The X-ray powder diffraction spectrum obtained by measuring the prepared white crystalline powder using Cu-Kα rays is similar to that of Example 1.
Embodiment 3
[0067] Embodiment 3: Preparation of fumaric acid Vonola monohydrate
[0068] (1) Take 100g of vonoprazan fumaric acid crude product, add a mixed solution (600ml) of tetramethylethylenediamine / water (25:1.1 by volume), stir (30 rpm) and dissolve for 1-2min, Activated carbon decolorization, suction filtration;
[0069] (2) Reduce the filtrate of step (1) to -2°C, add dropwise (2.0mL / min) 480ml of pre-cooled tetramethylethylenediamine, and continue to cool down (3°C per 10 minutes) to -8°C Crystallization;
[0070] (3) Insulate and stir until the crystallization is complete, grow the crystal for 1 hour, filter with suction, wash with water, and dry at 45°C to obtain a white crystalline powder.
[0071] The X-ray powder diffraction spectrum obtained by measuring the prepared white crystalline powder using Cu-Kα rays is similar to that of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com