Application of activity-controllable polymers to regulation and control of chirality of leucine-lysine methacrylate copolymers
A technology of lysine methacrylate and leucine methacrylate, applied in the preparation of cationic chiral amino acid methacrylate polymer and its antibacterial application field, can solve the problem of poor biocompatibility and weak antibacterial activity And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
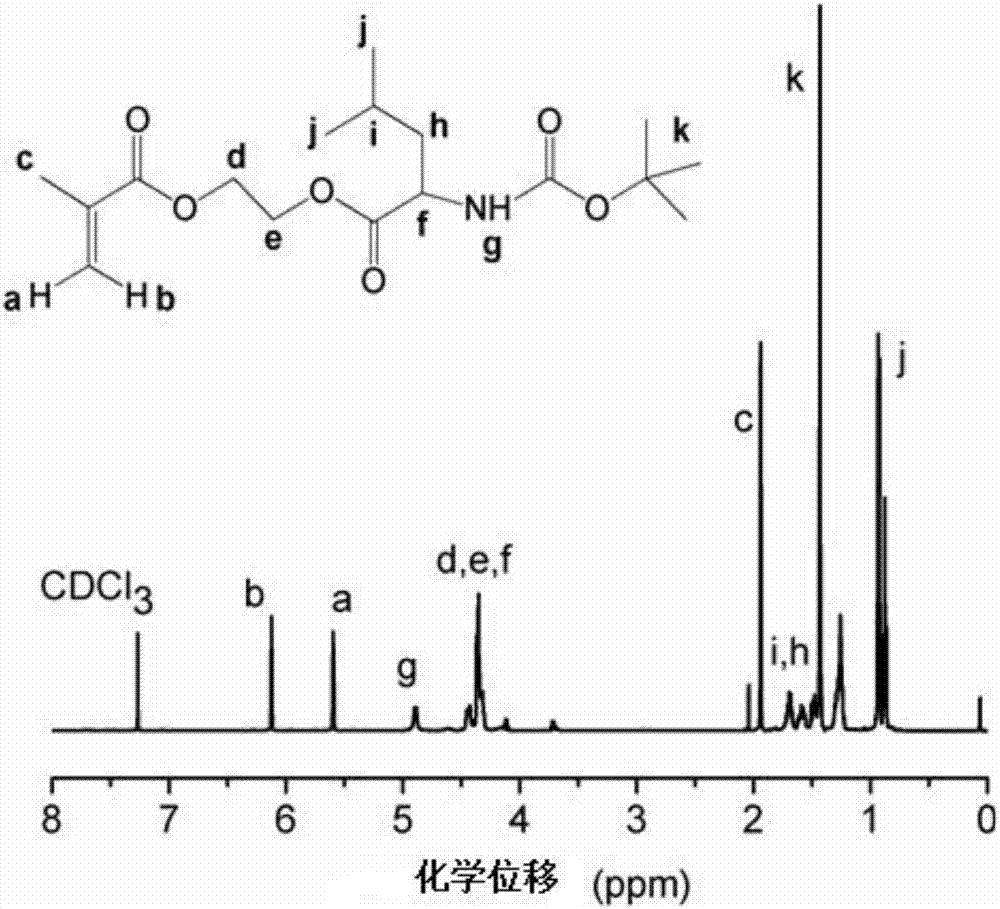
[0087] Example 1—Preparation of monomers D-leucine hydroxyethyl methacrylate, L-leucine hydroxyethyl methacrylate, D-lysine hydroxyethyl methacrylate and L-lysine methyl Hydroxyethyl Acrylate
[0088] First, add 5 g of chiral amino acid monomers (D-leucine, L-leucine, D-lysine, and L-lysine) dissolved in 22 mL of dry dichloromethane into a dry double-neck round bottom flask. acid, in order to ensure the activity of the functional group in the reaction, select the above four amino acids protected by tert-butoxycarbonyl Boc, and use trifluoroacetic acid to remove the Boc protecting group after the reaction), purify with nitrogen gas under magnetic stirring, and then add soluble in 1.5 0.24g of catalyst DMAP in mL of dry dichloromethane, put the reaction flask in an ice-water bath, slowly add 4.53g of dehydration condensation agent DCC dissolved in 20mL of dry dichloromethane dropwise, and add 2.86g of it within 20min under the protection of nitrogen HEMA. The reaction was carr...
Embodiment 2
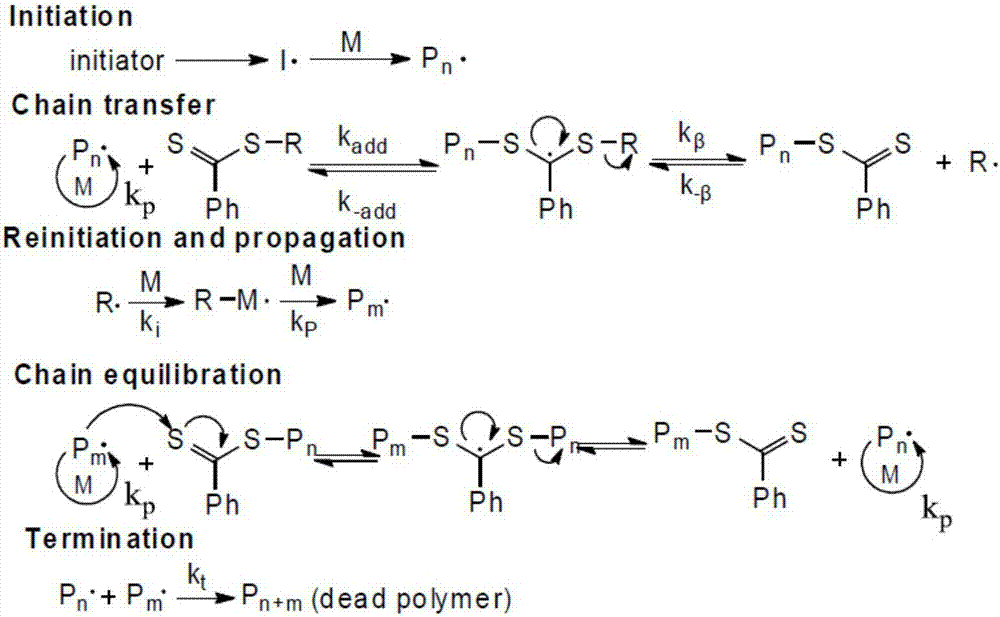
[0090] Example 2—Using the four monomers prepared in Example 1 as raw materials, four homopolymers were prepared using reversible addition-fragmentation chain transfer polymerization
[0091] Prepare cationic chiral amino acid hydroxyethyl methacrylate homopolymer by RAFT polymerization method: in a 25mL Schlenk bottle with a magnetic stirrer, add 1.5g of the monomer prepared in Example 1, CPADB 24.4mg, AIBN 2.86mg and 1.5 g of anhydrous DMF solvent, after three freeze-pump-thaw cycles to remove impurity gases in the reaction system, placed in an oil bath at 70° C., and reacted for 10 hours under nitrogen protection. After the reaction was completed, it was exposed to the air and placed in an ice-water bath for rapid cooling to terminate the reaction, and then the acetone / n-hexane precipitation was repeated 5 times. The obtained product was dried in a vacuum oven at 30° C. for 8 hours to obtain a sample of each homopolymer. Under the condition of ice-water bath, according to ...
Embodiment 3
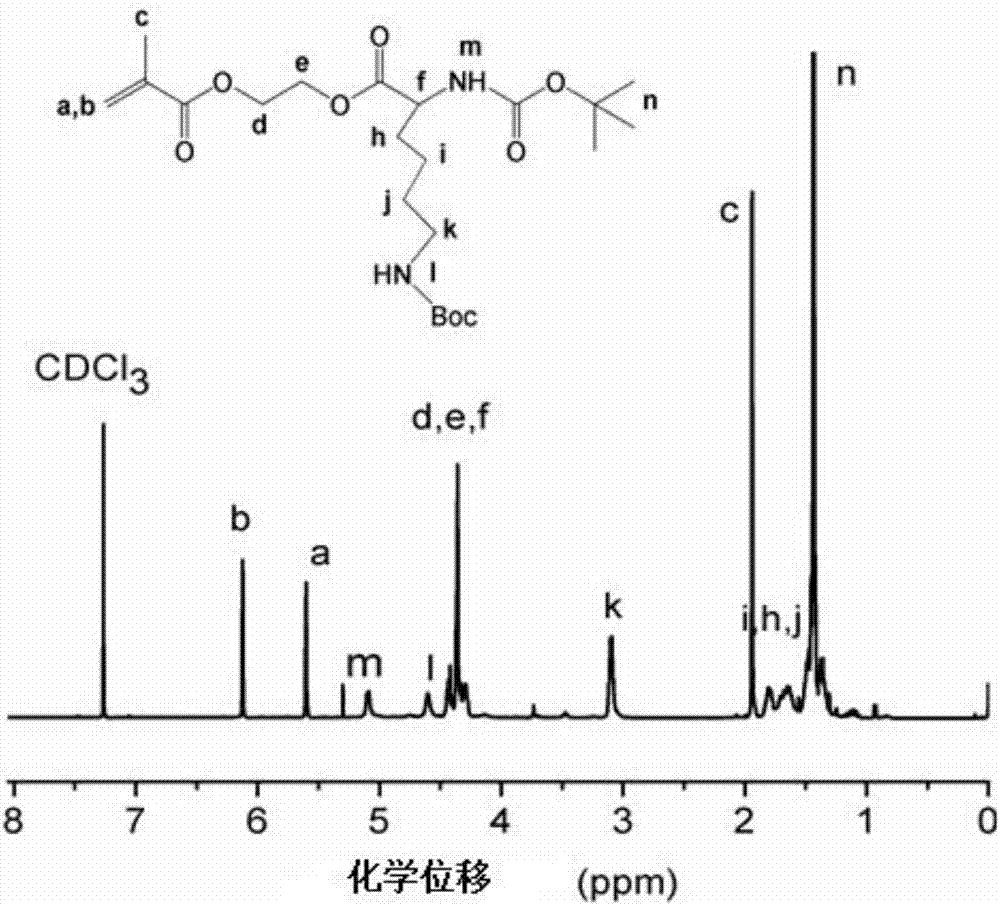
[0093] Example 3—Preparation of D-cationic chiral amino acid hydroxyethyl methacrylate copolymer
[0094] (1) Adopt the method of embodiment 2 to prepare the homopolymer of D-leucine at first: in the 25mLSchlenk bottle with magnetic stirrer, add the monomer D-leucine methacrylic acid prepared by 1.5g embodiment 1 Hydroxyethyl ester, CPADB 24.4mg, AIBN 2.86mg and 1.5g of anhydrous DMF solvent, after three freeze-pump-thaw cycles to remove impurity gases in the reaction system, put them in an oil bath at 70°C under nitrogen protection React for 10 hours, after the reaction is basically finished, do not carry out the termination reaction of exposing the air, but keep the active group (CPADB) at the end of the D-leucine homopolymer as a macromolecular chain transfer for adding the second monomer to react agent;
[0095] (2) In the reaction vessel, add 3g of the same configuration of lysine methacrylate monomer (i.e. D-lysine hydroxyethyl methacrylate), AIBN 2.15mg and 3g of anhyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com