Nickel-cobalt compound hydroxide and method and device for producing same
一种复合氢氧化物、制造方法的技术,应用在镍化合物、化学电极制造、化学仪器和方法等方向,能够解决充放电寿命降低、难工业规模的生产、自放电强烈等问题,达到提高循环特性、减少成本、提高生产效率的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
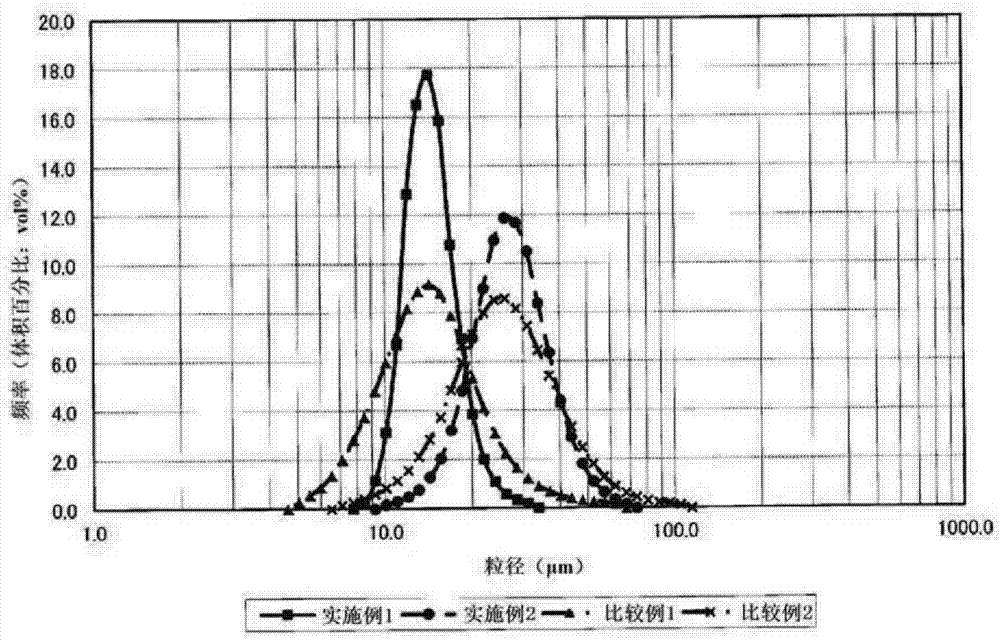
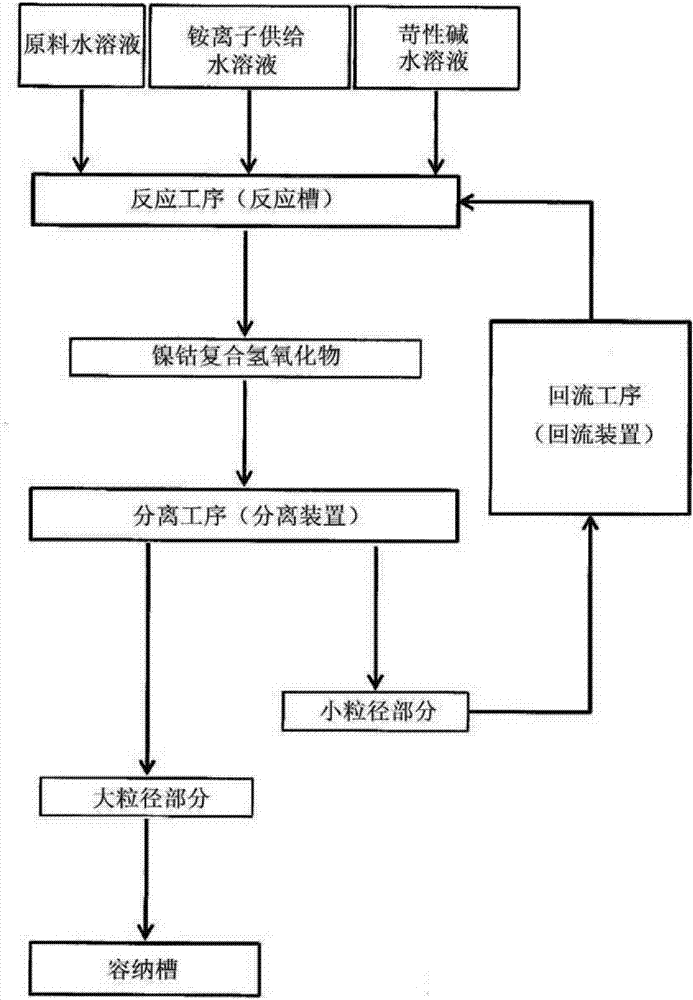
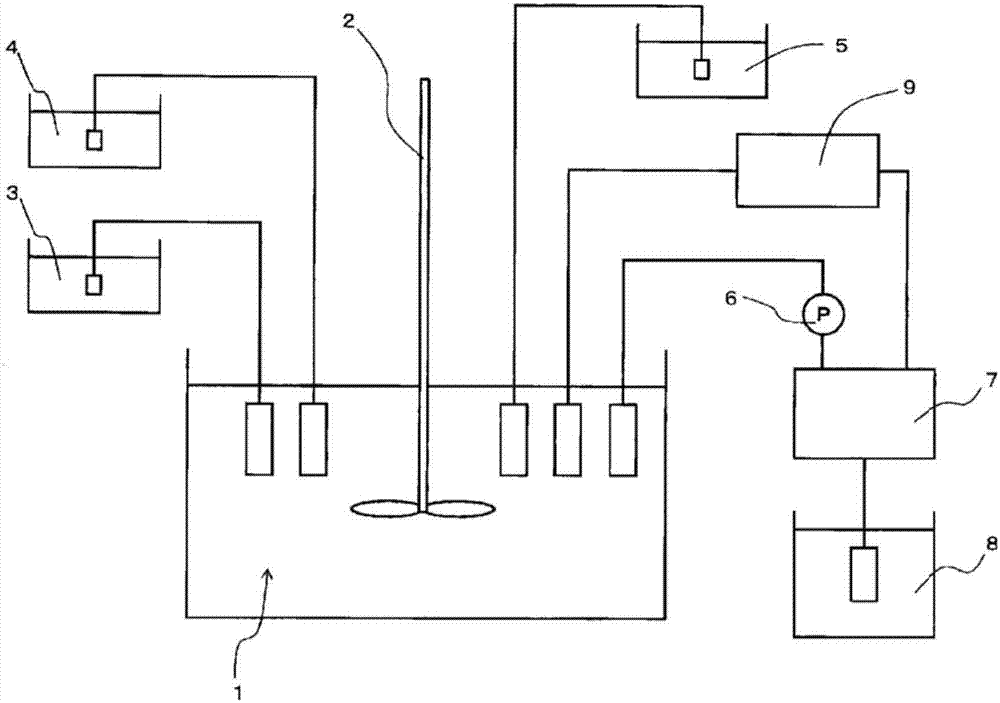
[0188] In an overflow crystallization reaction tank with a volume of 34L equipped with four baffles, 32L of industrial water and 1300mL of 25% by mass ammonia water were put in, and heated to 50°C through a constant temperature bath and a heating mantle. Thereafter, a 24% by mass caustic soda aqueous solution was injected, and the pH was controlled to 12.2 based on a liquid temperature of 25°C. Specifically, in order to correctly implement the management of the pH value, the pH value at a temperature of 50° C. is controlled so that the pH value at a temperature of 25° C. is between 12.1 and within the scope of 12.3.
[0189] The crystallization reaction was carried out in the following manner: in a reaction tank maintained at 50°C, while stirring, a constant pump was used to supply 30 mL / minute (min) to adjust the molar concentration of Ni to 1.69 mol / L and the molar concentration of Co to 0.31 mol / L raw material aqueous solution, and while supplying 25 mass % ammonia water a...
Embodiment 2
[0197] The pH of the reaction aqueous solution was maintained at 11.8 based on the liquid temperature of 25° C., and the reaction was carried out at a stirring speed of 800 rpm in the reaction step, and a composite hydroxide was obtained in the same manner as in Example 1. In addition, the management of the pH value is as follows: the reaction aqueous solution in the reaction tank is cooled to 25°C and the pH value is measured, thereby controlling the pH value at a temperature of 50°C so that the pH value at a temperature of 25°C is in the range of 11.7 to 11.9 Inside. The obtained composite hydroxide is subjected to appropriate solid-liquid separation, washed with water and dried to obtain a powdery composite hydroxide. As a result of ICP emission spectroscopic analysis of this composite hydroxide, it was confirmed that it has the general formula: Ni 0.85 co 0.15 (OH) 2 Expressed. In addition, the composite hydroxide was carried out in the same manner as in Example 1 to m...
Embodiment 3
[0202] The composite hydroxide was obtained in the same manner as in Example 1, except that the pH of the reaction aqueous solution was maintained at 12.0 based on the liquid temperature of 25°C. As a result of ICP emission spectroscopic analysis of this composite hydroxide, it was confirmed that it has the general formula: Ni 0.85 co 0.15 (OH) 2 Expressed. In addition, the composite hydroxide was carried out in the same manner as in Example 1 to measure the particle size distribution (D10, D50, and D90) and the tap density. The results are shown in Table 1.
[0203] Thereafter, a composite oxide covered with aluminum was synthesized in the same manner as in Example 1. As a result of ICP emission spectroscopic analysis of this composite oxide, it was confirmed that it has the general formula: Ni 0.83 co 0.13 al 0.04 o 2 Expressed.
[0204] In addition, it carried out similarly to Example 1, and obtained the positive electrode active material. As a result of ICP emiss...
PUM
| Property | Measurement | Unit |
|---|---|---|
| actual density | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| actual density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com