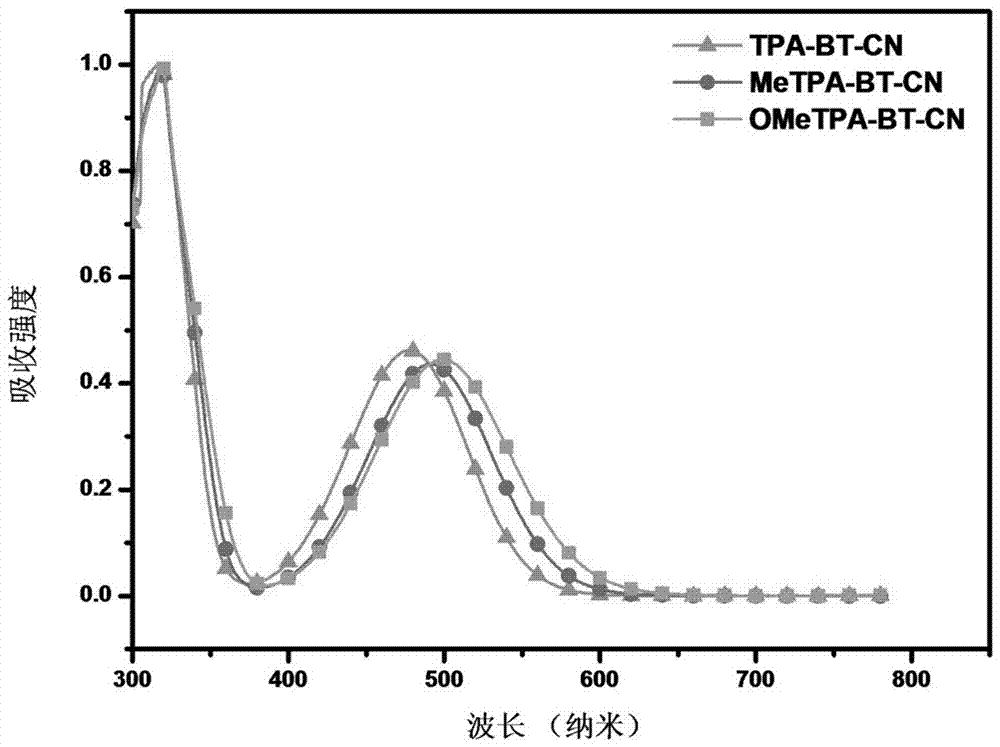
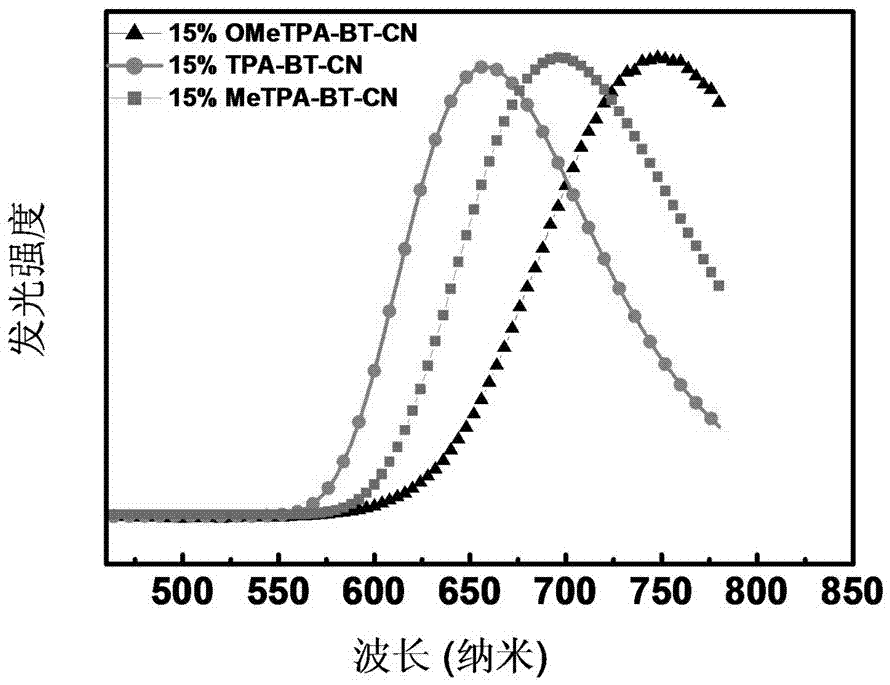
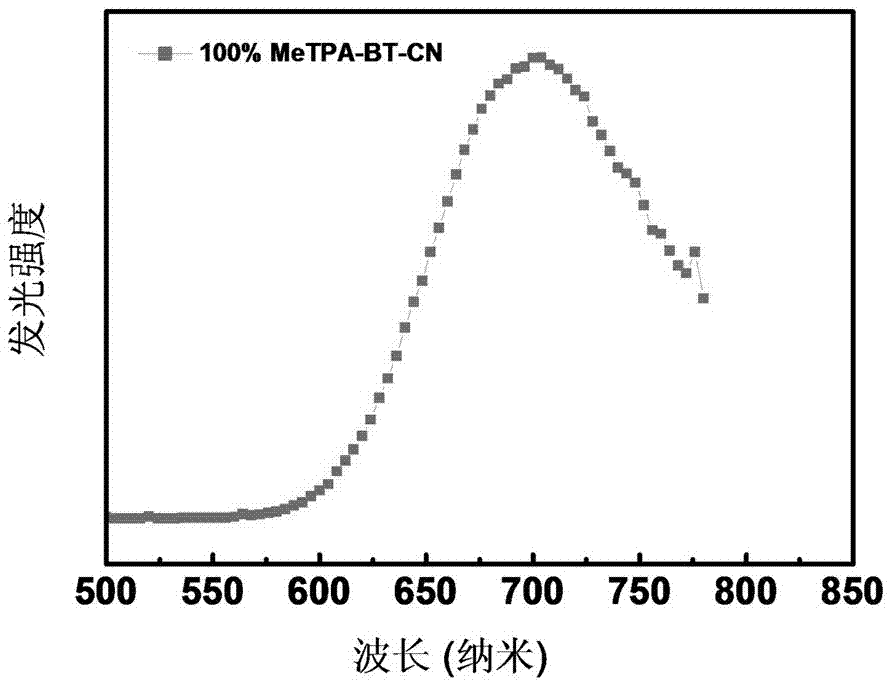
2,1,3-Benzothiadiazole-based D-A-A type near-infrared luminous compound and application thereof
A technology of benzothiadiazole and light-emitting compound, which is applied in the field of D-A-A type near-infrared light-emitting compound, and achieves the effect of improving efficiency roll-off, excellent electroluminescence effect and small efficiency roll-off
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Step 1: Dissolve 3.7 g of triphenylamine borate, 2.43 g of 7-bromo-4-formyl benzo[C][1,2,5]thiadiazole in 100 mL of water and 1, In the mixed solution of 4-dioxane, wherein the volume ratio of water and 1,4-dioxane is 1:10, and 3.45 grams of potassium carbonate and 8% equivalent tetrakistriphenylphosphine palladium are added to the reaction in the bottle. After reflux for 48 hours under the protection of argon, the reaction solution was cooled to room temperature. The solvent was removed to dryness by a rotary evaporator. The reaction solid was dissolved in 80 mL of dichloromethane, and the organic layer was washed three times with 50 mL of water. The organic layer was dried over anhydrous sodium sulfate and spin-dried. Add silica gel and spin-dry the resulting solid to pass through the column with dichloromethane / petroleum ether at a ratio of 4:6 (volume ratio), and spin-dry to obtain 3.45 g of 7-triphenylamine-4-formylbenzo[C][1,2,5 ] Thiadiazole, productive rate ...
Embodiment 2
[0035] Step 1: Put 4.00 g of 4,4'-dimethyltriphenylamine borate and 2.43 g of 7-bromo-4-formylbenzo[C][1,2,5]thiadiazole under the protection of argon Dissolve in a mixed solution of 100 mL of water and 1,4-dioxane, wherein the volume ratio of water and 1,4-dioxane is 1:10, and add 3.45 grams of potassium carbonate and 8% equivalent Tetraphenylphosphine palladium into the reaction flask. After reflux for 48 hours under the protection of argon, the reaction solution was cooled to room temperature. The solvent was removed to dryness by a rotary evaporator. The reaction solid was dissolved in 80 mL of dichloromethane, and the organic layer was washed three times with 50 mL of water. The organic layer was dried over anhydrous sodium sulfate and spin-dried. Add silica gel and spin-dry the resulting solid to pass through the column with dichloromethane / petroleum ether at a ratio of 5:5 (volume ratio), and spin-dry to obtain 3.84 g of 7-(4,4'-dimethyltriphenylamine)-4-formylbenzen...
Embodiment 3
[0038] Step 1: 4.3 g of 4,4'-dimethoxytriphenylamine borate, 2.43 g of 7-bromo-4-formyl benzo[C][1,2,5]thiadiazole under argon protection Dissolve in a mixed solution of 100 mL of water and 1,4-dioxane, wherein the volume ratio of water and 1,4-dioxane is 1:10, and add 3.45 grams of potassium carbonate and 8% equivalent Tetraphenylphosphine palladium into the reaction flask. After reflux for 48 hours under the protection of argon, the reaction solution was cooled to room temperature. The solvent was removed to dryness by a rotary evaporator. The reaction solid was dissolved in 80 mL of dichloromethane, and the organic layer was washed three times with 50 mL of water. The organic layer was dried over anhydrous sodium sulfate and spin-dried. Add silica gel and spin-dry the resulting solid to pass through the column with dichloromethane / petroleum ether at a ratio of 3:7 (volume ratio), and spin-dry to obtain 3.85 g of 7-(4,4'-dimethoxytriphenylamine)-4-aldehyde Benzo[C][1,2,5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com