Solid phase synthetic method of liraglutide
A solid-phase synthesis method, the technology of liraglutide, which is applied in the preparation methods of peptides, peptides, specific peptides, etc., can solve the problems of complex synthesis steps and difficult purification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] (1) Preparation of Polypeptide Resin I
[0070] Weigh 57.1 grams of Fmoc-Gly-King resin (0.35mmol / g, 20mmol), soak in 500ml DMF for 30 minutes, drain, add 500ml decapping reagent, react for 30 minutes, drain, wash 5 times with DMF, add Fmoc- Arg(Pbf)-OH (MW: 648.8, 40mmol) 26.0g, HBTU (MW: 379.2, 40mmol) 15.2g, HOBT (MW: 135.1, 40mmol) 5.4g, NMM (MW: 102.1, 80mmol) 9.0ml, DMF500ml , reacted for 0.5-2 hours, ninhydrin detection resin was colorless and transparent, drained, washed 3 times with DMF, drained to obtain Fmoc-Arg(Pbf)-Gly-King resin, and then added deprotection reagent, deprotection Reaction, then add the amino acid with Fmoc protection group, and so on, until the 12th lysine is connected, DMF washes 3 times to obtain the polypeptide resin I: Dde-Lys(mmt)-Glu(Otbu)-Phe- Ile-Ala-Trp(Boc)-Leu-Val-Arg(Pbf)-Gly-Arg(Pbf)-Gly-Wang resin.
[0071] The amount of amino acid added in each step of condensation reaction is respectively:
[0072] Fmoc-Gly-OH (MW: 297.3,...
Embodiment 2
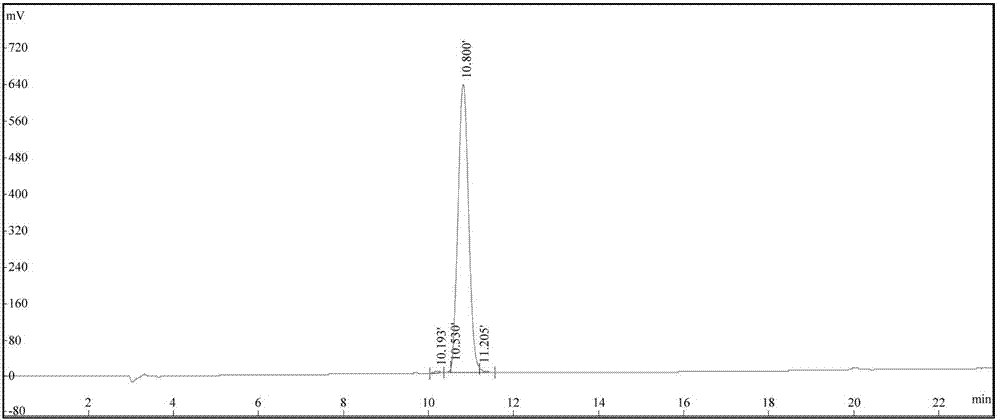
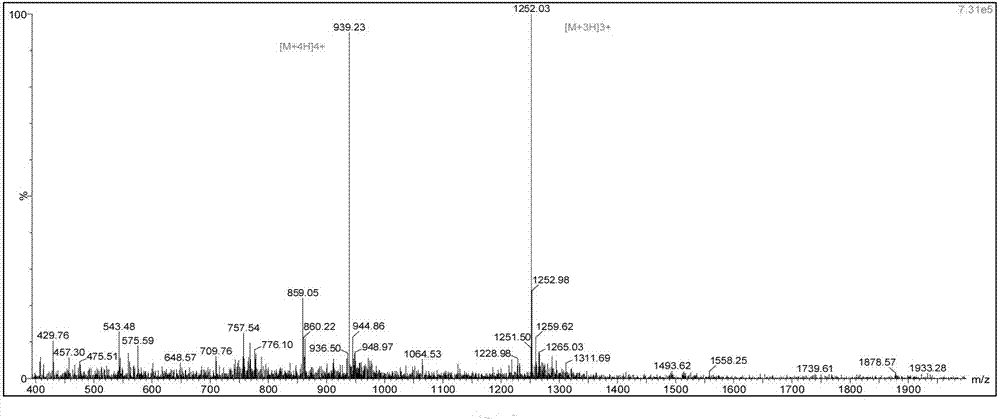
[0115] The degree of substitution of the selected Fmoc-Gly-King resin was 0.3mmol / g; the rest was the same as in Example 1, and finally 37g of Liraglutide fine product was obtained, with a total yield of 50%.
Embodiment 3
[0117] The degree of substitution of the selected Fmoc-Gly-King resin was 0.5mmol / g; the rest was the same as in Example 1, and finally 35g of liraglutide was obtained, with a total yield of 46%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com