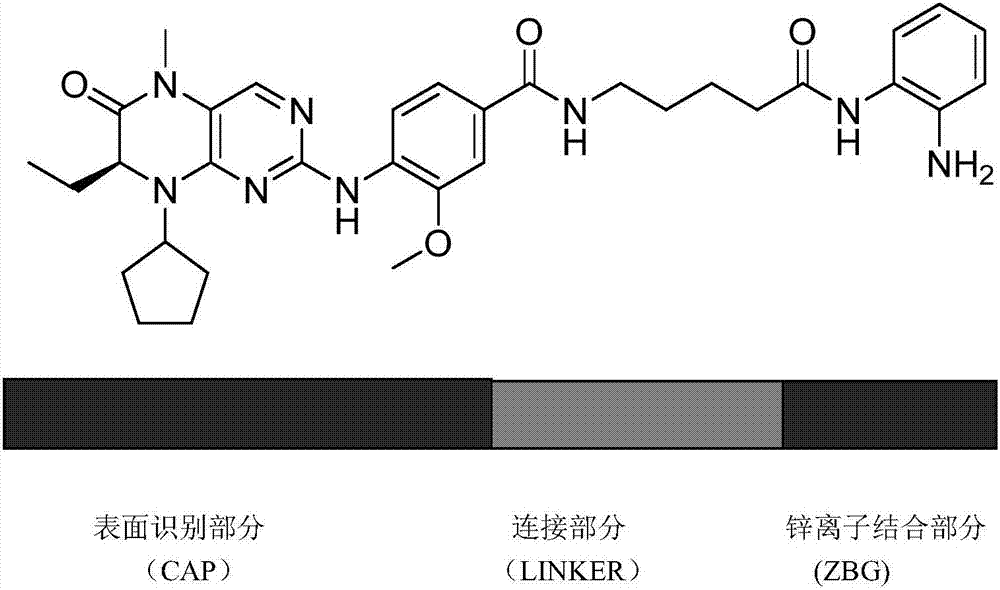
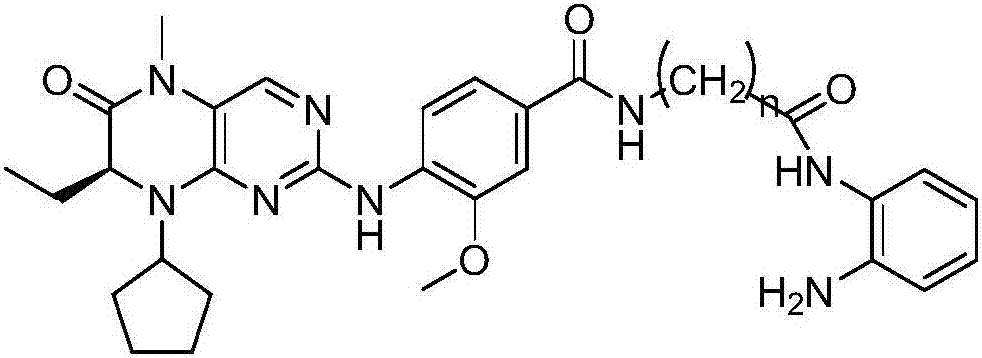
BET (bromodomain and extraterminal domain)/HDAC (histone deacetylase) double-target inhibitor, and preparation method and application thereof
An inhibitor and dual-target technology, applied in the field of medicine and chemical industry, can solve the problems of no BI-2536 derivative inhibitor report, etc., and achieve the effects of high yield, simple preparation method and improved therapeutic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
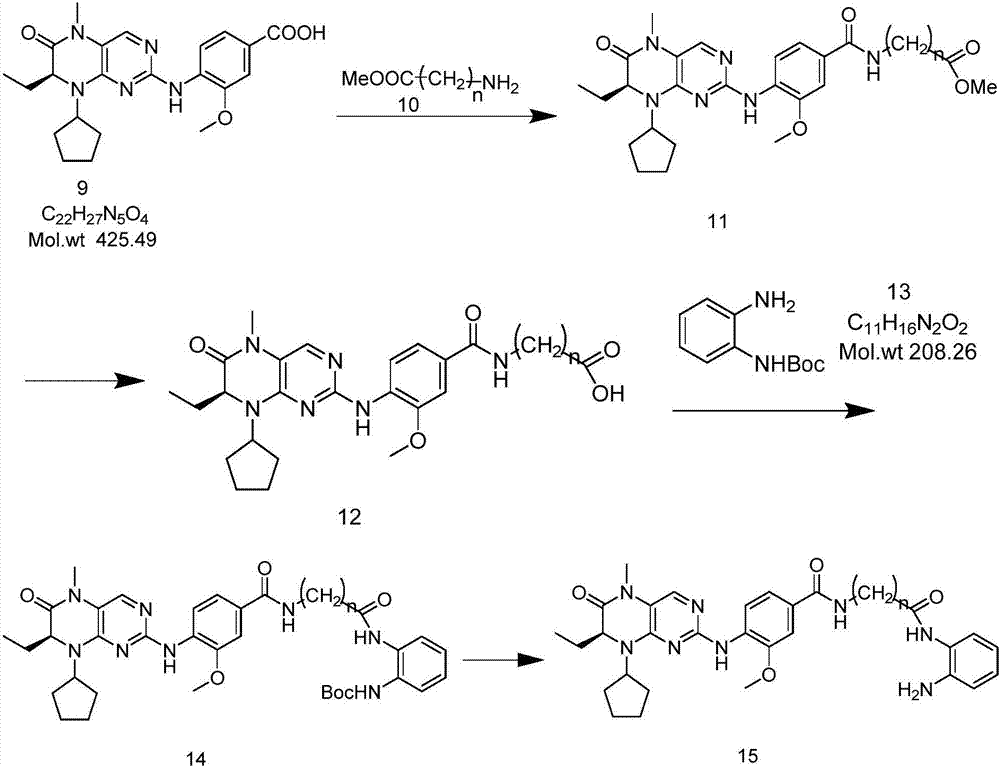
[0046] Take the synthesis of 15c (BI-C) as an example below to further illustrate the present invention:
[0047] 1. Preparation of compound 2, the reaction formula is as follows:
[0048]
[0049] Compound 1 (1g, 9.96mmol) was weighed and dissolved in 10mL of methanol, and thionyl chloride (1.48ml, 20.36mmol) was slowly added in an ice-water bath at 0°C, refluxed at 65°C for 1.5h, and distilled under reduced pressure after the reaction. The remaining oil was mixed with 10ml of methyl tert-butyl ether and stirred for 0.5h. The resulting colorless crystals were filtered, washed with ether, and dried in vacuo overnight to obtain colorless semi-solid crystals, namely the target compound 2 (1.12g, quant.).
[0050] The target product compound 2 1 The data of H NMR are as follows:
[0051] 1H NMR (300MHz, CDCl 3 ) δ: 8.71 (s, 2H), 4.20-4.01 (m, 1H), 3.66 (s, 3H), 2.24-2.02 (m, 2H), 1.09 (t, J=7.0Hz, 3H).
[0052] 2. Preparation of compound 4, the reaction formula is as follo...
example 5-1
[0071] Weigh compound 7 (280mg, 1mmol) and dissolve it in 5ml N,N-dimethylformamide solution, add iodomethane (80μl, 1.3mmol), cool the reaction to -10°C, add 60% Sodium hydride (52mg, 1.3mmol) was reacted at 0°C for 30min, raised to room temperature and reacted for 3h, the plate was spotted to confirm the completion of the reaction, and crushed ice was added to terminate the reaction. Extracted twice with ethyl acetate, washed with water, dried the organic phase with anhydrous magnesium sulfate, filtered, distilled under reduced pressure, and column chromatography gave light yellow compound 8 (294mg, quant.)
[0072] The target product compound 8 1 The data of H NMR are as follows:
[0073] 1 H NMR (300MHz, CDCl 3 )δ:
[0074] 7.67 (s, 1H), 4.38-4.30 (m, 1H), 4.24 (dd, J=7.47Hz, 3.6Hz, 1H), 3.33 (s, 3H), 2.08-2.02 (m, 1H).
example 5-2
[0076] Weigh compound 7 (280 mg, 1 mmol) and dissolve it in 5 ml of tetrahydrofuran solution, add iodomethane (80 μl, 1.3 mmol), cool the reaction to -10 ° C, add 60% sodium hydride (52 mg, 1.3 mmol) dispersed in mineral oil React at 0°C for 30 min, rise to room temperature for 3 h, spot the plate to confirm the end of the reaction, and add crushed ice to terminate the reaction. Extracted twice with ethyl acetate, washed with water, dried the organic phase with anhydrous magnesium sulfate, filtered, distilled under reduced pressure, column chromatography gave light yellow compound 8 (235 mg, yield 80%)
[0077] 6. The synthesis of compound 9, the reaction formula is as follows:
[0078]
[0079] Weigh compound 8 (235mg, 0.8mmol) and 4-amino-3-methoxybenzoic acid (208mg, 1.24mmol) into a mixed solvent of 0.6ml ethanol, 2.4ml water, 260μl concentrated hydrochloric acid, and reflux the reaction mixture at 95°C for 48h . Distillation under reduced pressure and column chromato...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com