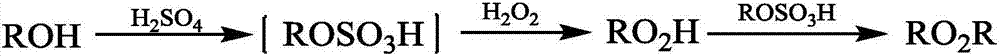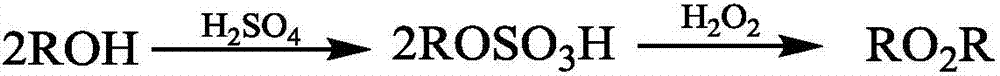Preparation method of di-tert-butyl peroxide
A technology of di-tertiary peroxide and di-tert-butyl peroxide, which is applied in the field of preparation of di-tertiary peroxide, can solve the problems of high safety risk, long reaction time, strict requirements on feeding temperature, etc. High performance, short reaction time and easy control of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] A kind of preparation method of di-tertiary base peroxide, tert-butanol, hydrogen peroxide, catalyzer are introduced in the micro-reaction device in continuous mode, and the total flow velocity that tert-butanol, hydrogen peroxide and catalyzer enters micro-reaction device is 5 ~30mL / min, that is, the sum of the flow rates of tert-butanol, hydrogen peroxide and catalyst is 5~30mL / min, so that tert-butanol and hydrogen peroxide undergo peroxidation reaction, the reaction temperature is 30~60℃, and the time is 26 ~218s, make the stream that comprises di-tert-butyl peroxide, then draw the stream that comprises di-tert-butyl peroxide from the micro-reaction device, separate, wash with water, dry to obtain di-tert-butyl peroxide, Wherein catalyzer is the sulfuric acid that mass concentration is 98%;
[0026] Wherein the molar ratio of tert-butanol to peroxide is 1:2~1:2.2, and the catalyst consumption accounts for 10~20% of the total mass of tert-butanol, hydrogen peroxide a...
Embodiment 1
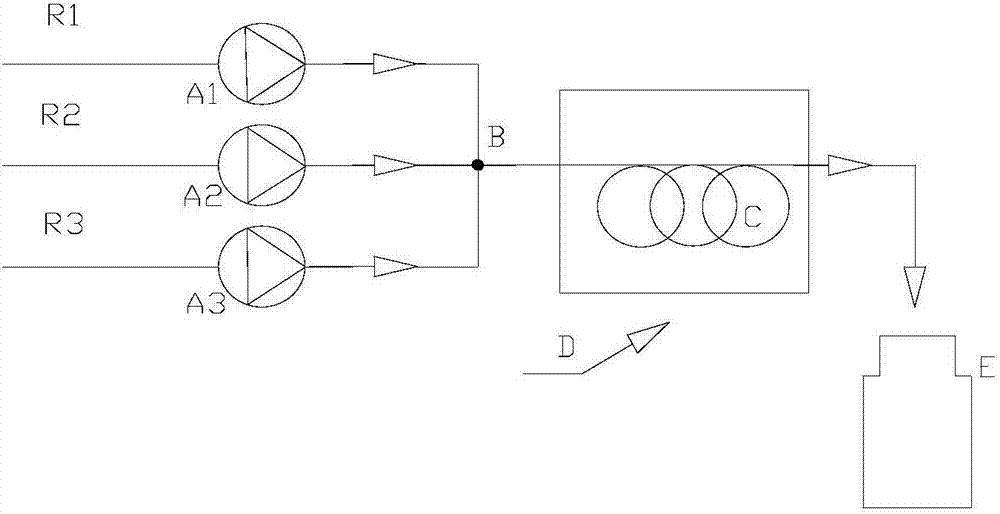
[0029] Such as figure 1 As shown, tert-butanol, hydrogen peroxide, and sulfuric acid are transported into the micro-mixer B by the first micro-advection pump A1, the second micro-advection pump A2, and the third micro-advection pump A3 (T-shaped stainless steel tee, inner diameter 0.5 mm), uniform mixing reaction, the total feed rate is 5mL / min, the mol ratio of tert-butanol to hydrogen peroxide is 1:2, and the sulfuric acid consumption is 10% of the overall material quality. The length of microreactor C is 10m, placed in thermostat D (30°C), and the reaction residence time is 26s. The material enters the product collector E after fully reacting in the microreactor. After the crude product was separated, washed with water and dried, the peroxide components were analyzed by gas chromatography. The content of di-tert-butyl peroxide was 89.7%, and the content of mono-tert-butyl peroxide was 10.2%. The conversion rate of tert-butanol is 98.1%.
Embodiment 2
[0031] Such as figure 1 As shown, tert-butanol, hydrogen peroxide, and sulfuric acid are transported into the micro-mixer B by the first micro-advection pump A1, the second micro-advection pump A2, and the third micro-advection pump A3 (T-shaped stainless steel tee, inner diameter 0.5 mm), uniform mixing reaction, the total feed rate is 5mL / min, the mol ratio of tert-butanol to hydrogen peroxide is 1:2.02, and the sulfuric acid consumption is 10% of the overall material quality. The length of microreactor C is 10m, placed in thermostat D (30°C), and the reaction residence time is 26s. The material enters the product collector E after fully reacting in the microreactor. After the crude product was separated, washed with water and dried, the peroxide components were analyzed by gas chromatography. The content of di-tert-butyl peroxide was 91.6%, and the content of mono-tert-butyl peroxide was 8.2%. The conversion rate of tert-butanol is 99.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com