Quality control method and fingerprint of spleen invigorating and kidney benefiting prescription
A quality control method, a technology of invigorating the spleen and kidney, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of complex components of traditional Chinese medicine and unclear active ingredients.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] A quality control method for invigorating the spleen and benefiting the kidney, comprising the following steps:
[0081] S1. Prepare the test solution of Bupi Yishen Fang;
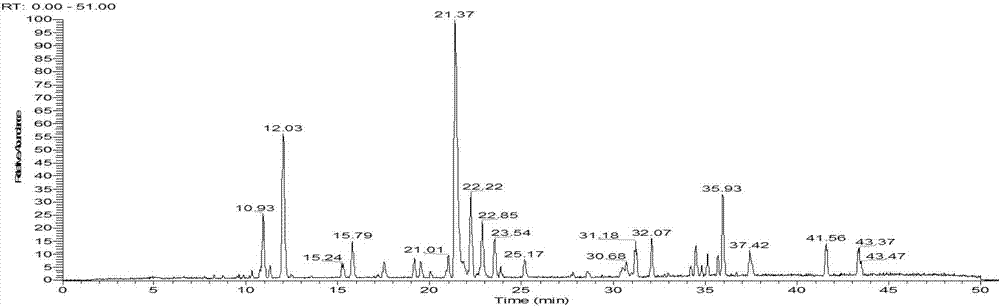
[0082] S2. draw need testing solution and inject liquid chromatograph, record chromatogram;
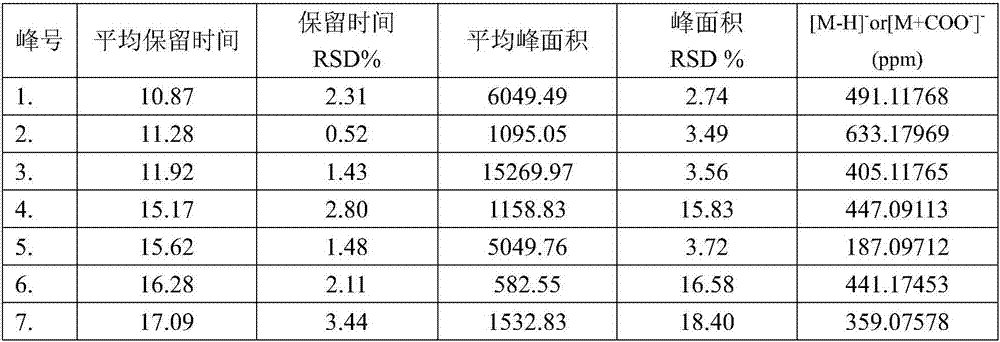
[0083] S3. Import the chromatogram obtained in step S2 into the Chinese medicine chromatographic fingerprint similarity evaluation system; select the chromatographic peaks that exist in the chromatograms of different batches of Bupi Yishen formula as common peaks; use the average value calculation method to generate Bupi Yi The standard fingerprint of Shenfang; calculate the relative retention time and relative peak area of each common peak;
[0084] S4. Establish the fingerprint of the Bupi Yishen formula to be detected; then compare it with the standard fingerprint described in step S3, and finally determine whether the quality of the Bupi Yishen formula to be tested is qualified;
[0085] The need te...
Embodiment 2
[0143] Further, after the liquid chromatograph described in embodiment 1 step S2, a mass spectrometer is also connected to detect the molecular weight of the common peak; the mass spectrometer adopts an electrospray ion source (ESI), and the sheath gas is 45 units , the auxiliary gas is 6 units, the spray voltage is -2.5~-3.5kV, the capillary temperature is 300~375°C, the sample is first scanned with high resolution, the second mass spectrometer is scanned with dynamic data dependence, and the collision energy is set to 20~ 45%.
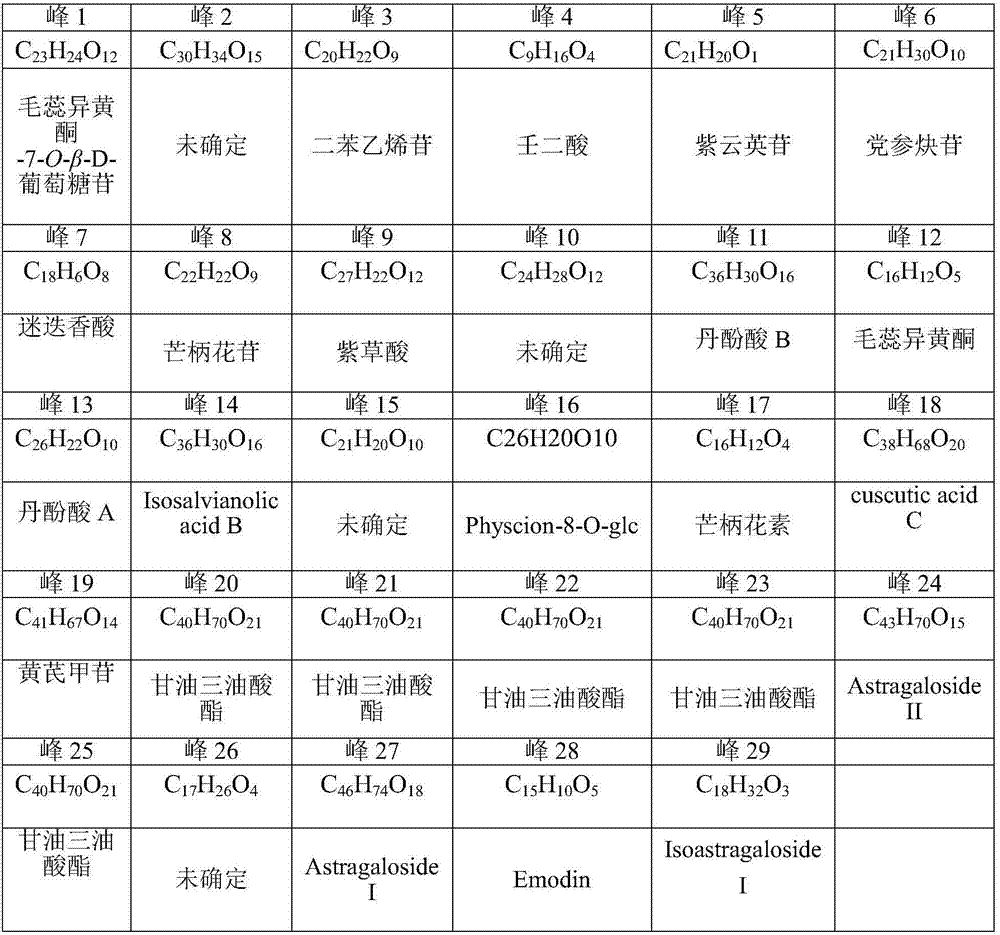
[0144] Further, on the basis of Example 1, calycosin-7-O-β-D-glucoside, formononetin, calycosin, stilbene glycoside, rosmarinic acid, shikonic acid, salvianolic acid A, salvianolic acid B, astragaloside, astragaloside, astragaloside, astragaloside II, salvianolic acid B, astragaloside, codonopside, formononetin, astragaloside II, etc., accurately weighed the appropriate amount, dissolved in 20% acetonitrile, prepared Standard solution, HPLC sample i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com