Short peptide for inhibiting tumors as well as coding gene and application of short peptide
A technology that encodes genes and tumors. It is applied in applications, anti-tumor drugs, and genetic engineering. It can solve problems such as structural instability, long peptide chains, and difficult chemical synthesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
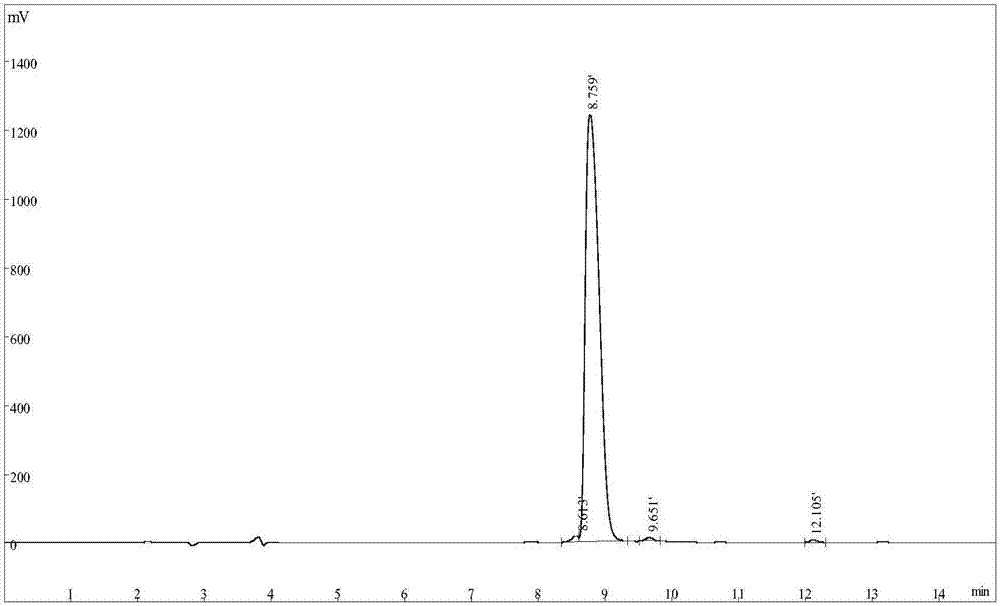
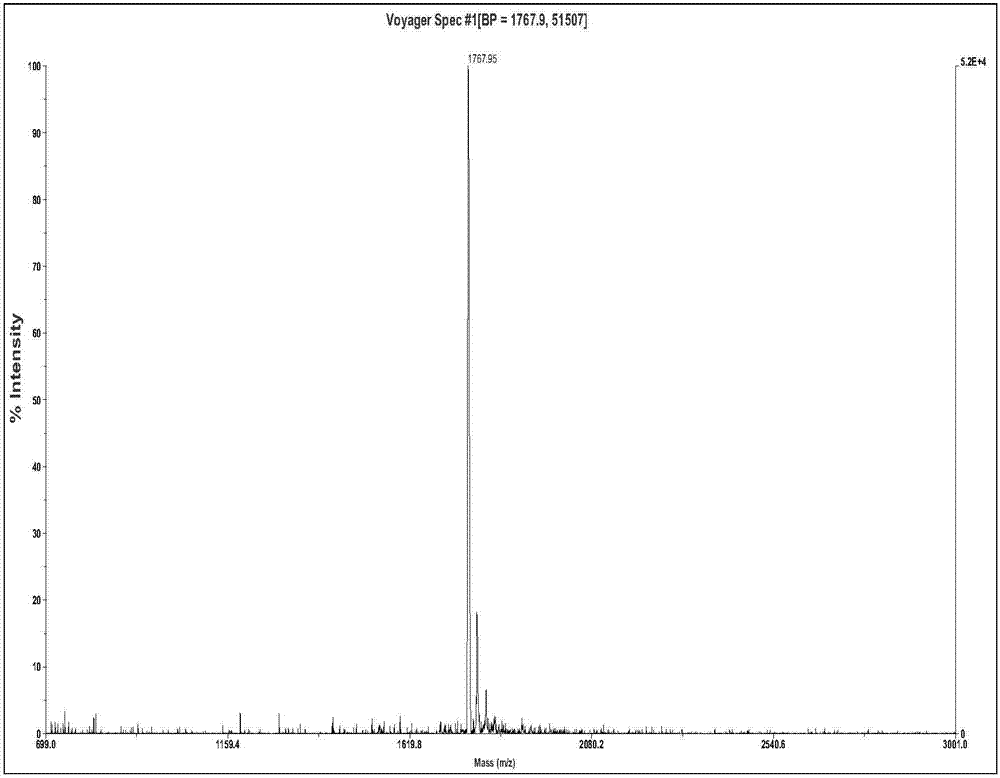
[0026] Example 1: Solid-phase synthesis, cleavage, purification and identification of tumor-inhibiting short peptides
[0027] The present invention utilizes a polypeptide synthesizer to synthesize the short peptide for inhibiting tumors by a solid-phase synthesis method:
[0028] 1) With DMF as the solvent, the concentration of various α-amino acids protected by Fmoc is 0.25M, the concentration of HBTU solution and HOBt solution is 0.33M, the concentration of piperidine solution is 200ml / L, and the concentration of DIEA solution is 174.2ml / L.
[0029] 2) Weigh 0.05mmol of Fmoc-Ala-Wang resin (functional group content 0.33mmol / g) into a solid-phase reactor, add 8ml of DCM to swell overnight, and remove the solvent under reduced pressure. Add 8ml of piperidine solution with a concentration of 200ml / L, react at room temperature for 5min, and drain; then add 8ml of piperidine solution with a concentration of 200ml / L, react for 20min at room temperature, and drain; wash with 8ml ...
Embodiment 2
[0038] Example 2: Determination of antitumor activity of short peptides that inhibit tumors
[0039] (1) Use human cervical cancer cells (HeLa) in logarithmic phase of growth, digest and resuspend the cells to obtain a cell suspension, and dilute the cells to 50,000 cells / mL. Add 100 µL to each well of the 96-well plate, put the paved 96-well plate in the incubator, and store at 37°C, 5% CO 2 Incubate overnight (>8 hours) under conditions.
[0040] (2) Use 0.01M pH7.4 PBS to dissolve the short peptide at 1 mg / mL. Dilute the short peptide sample to 50 µg / mL with complete medium. Discard the cell culture medium cultivated overnight, add 100 µL of 50 µg / mL polypeptide to each well, and repeat 3 times in 4 parallels to each well. At the same time, an equal volume of 50 μg / ml 5-FU and 0.01M pH7.4 PBS were used as positive and negative controls, at 5%37 o C conditions were cultured for 18 h.
[0041] (3) Next, discard the culture medium and dry it, add 50 μL of ice-cold 500 g / L...
Embodiment 3
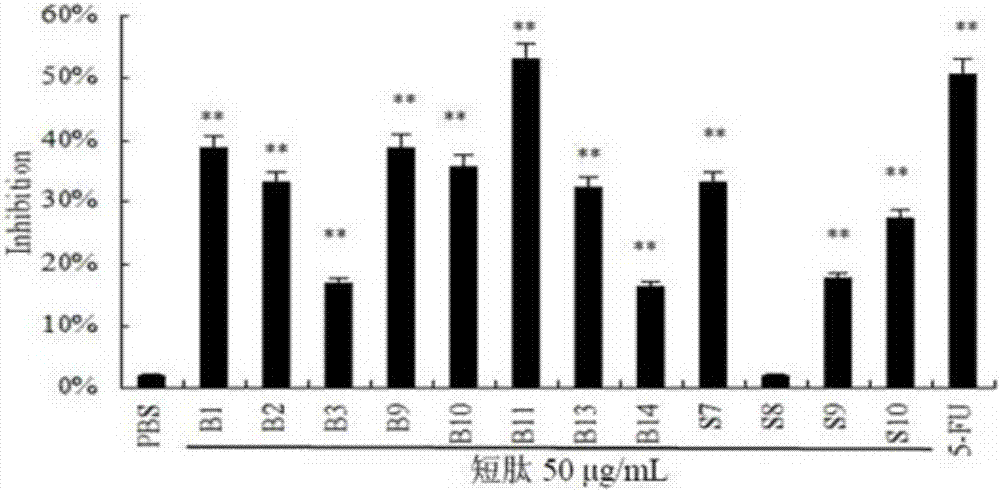
[0046] Example 3: Comparison of the antitumor activity of short peptides that inhibit tumors and other short peptide fragments in hemocyanin
[0047] (1) Synthesize 12 different hemocyanin short peptide fragments and name them respectively (B1, B2, B3, B9, B10, B13, B14, S7, S8, S9, S10). The short peptides of the present invention are named B11 .
[0048] (2) Use human cervical cancer cells (HeLa) in the logarithmic phase of growth, digest and resuspend the cells to obtain a cell suspension, and dilute the cells to 50,000 cells / mL. Add 100 µL to each well of the 96-well plate, put the paved 96-well plate in the incubator, and store at 37°C, 5% CO 2 Incubate overnight (>8 hours) under conditions.
[0049] (3) Use 0.01M pH7.4 PBS to dissolve the short peptide at 1mg / mL. Dilute different short peptide samples to 50 µg / mL with complete medium. Discard the cell culture medium cultivated overnight, add 100 µL of 50 µg / mL polypeptide to each well, and repeat 3 times in 4 paralle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com