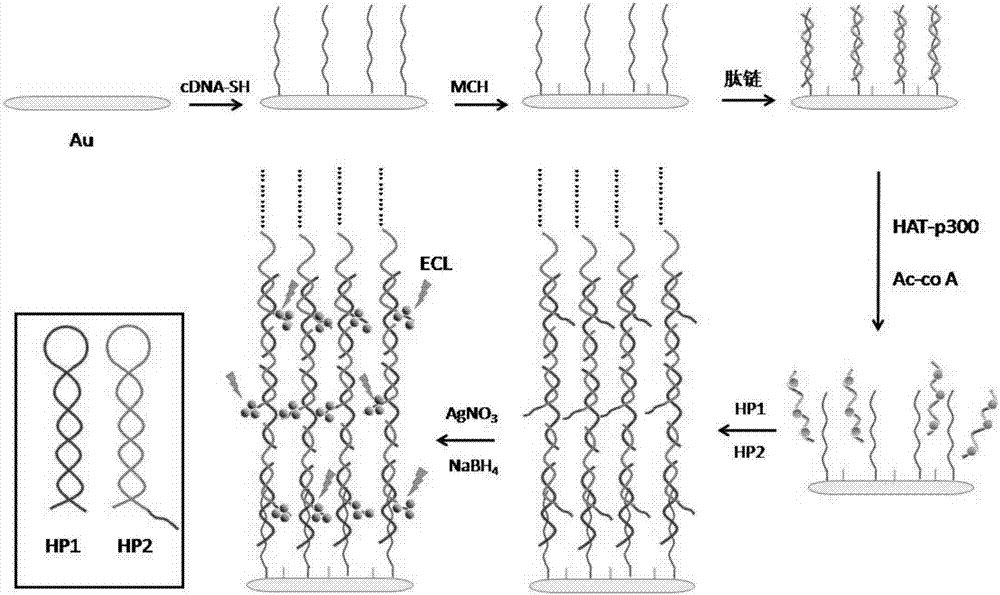
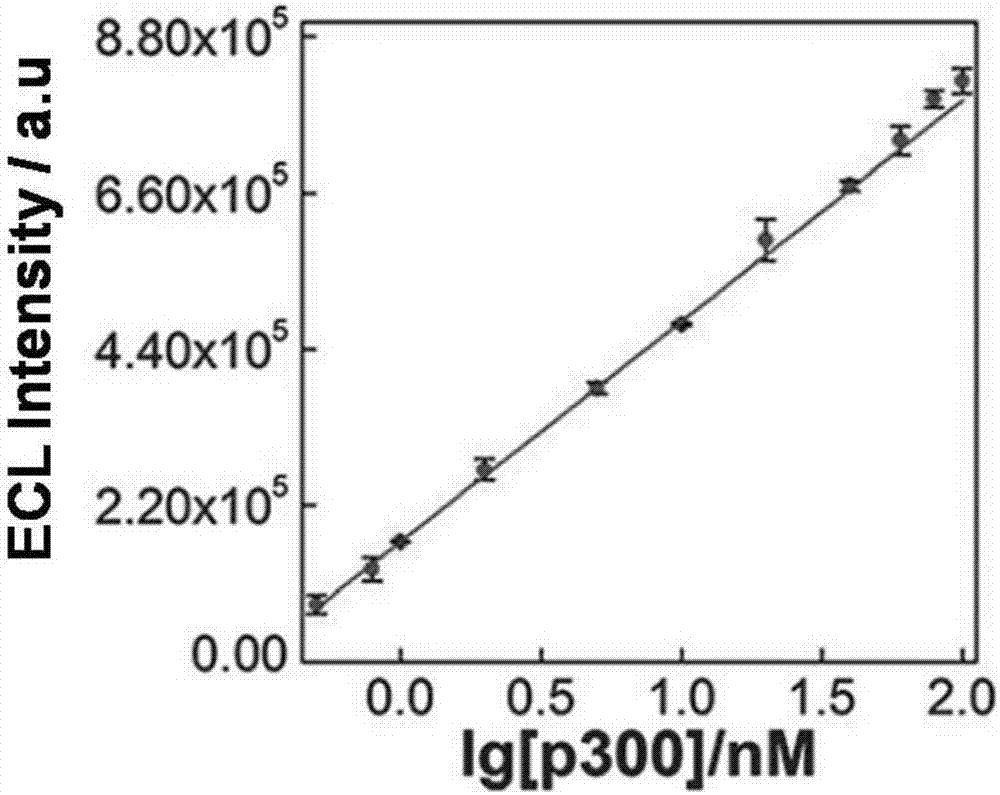
Construction of electrochemical luminescence sensor for detecting acetyltransferase activity
An acetyltransferase and activity detection technology, applied in the field of electrochemiluminescent biosensors, can solve the problems of no sensors yet, and achieve the effects of high detection sensitivity, enhanced signal strength, and easy manipulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] (1) Gold electrode surface pretreatment and activation
[0059] The gold electrode was made of 0.3 μm Al 2 o 3 The powder was ground and polished on the suede, then ultrasonically cleaned with ethanol and water for 3 minutes, and the surface of the electrode was blown dry with pure nitrogen.
[0060] Cleaned and dried gold electrode was used as working electrode, Ag / AgCl was used as reference electrode, and platinum wire was used as counter electrode. 2 SO 4 In solution, -0.20~1.65V, 100mV / s, scan CV until stable.
[0061] Repeat this until the gold electrode reaches the activation standard, wash the gold electrode with water, and dry it with nitrogen.
[0062] (2) Gold electrode surface modification
[0063] Dip the activated gold electrode into 1 μM cDNA (5'-CTA AGT AAC TCT GCA CTC TTA TAT ATCATA GAA TTG GTA GAT-(CH 2 )6-SH-3') in PBS solution (50mM, pH 7.4), incubated at 35°C for 1h to form a cDNA-modified Au electrode (cDNA-Au).
[0064] After washing with wa...
Embodiment 2
[0070] (1) Gold electrode surface pretreatment and activation
[0071] The gold electrode was made of 0.3 μm Al 2 o 3 The powder was ground and polished on the suede, then ultrasonically cleaned with ethanol and water for 2 minutes, and the surface of the electrode was blown dry with pure nitrogen.
[0072] Cleaned and dried gold electrode was used as working electrode, Ag / AgCl was used as reference electrode, and platinum wire was used as counter electrode. 2 SO 4In solution, -0.20~1.65V, 100mV / s, scan CV until stable.
[0073] Repeat this until the gold electrode reaches the activation standard, wash the gold electrode with water, and dry it with nitrogen.
[0074] (2) Gold electrode surface modification
[0075] Dip the activated gold electrode into 5 μM cDNA (5'-CTA AGT AAC TCT GCA CTC TTA TAT ATCATA GAA TTG GTA GAT-(CH 2 )6-SH-3') in PBS solution (50mM, pH 7.4), incubated at 37°C for 1h to form a cDNA-modified Au electrode (cDNA-Au).
[0076] After washing with wat...
Embodiment 3
[0082] (1) Gold electrode surface pretreatment and activation
[0083] The gold electrode was made of 0.3 μm Al 2 o 3 The powder was ground and polished on the suede, then ultrasonically cleaned with ethanol and water for 2 minutes, and the surface of the electrode was blown dry with pure nitrogen.
[0084] Cleaned and dried gold electrode was used as working electrode, Ag / AgCl was used as reference electrode, and platinum wire was used as counter electrode. 2 SO 4 In solution, -0.20~1.65V, 100mV / s, scan CV until stable.
[0085] Repeat this until the gold electrode reaches the activation standard, wash the gold electrode with water, and dry it with nitrogen.
[0086] (2) Gold electrode surface modification
[0087] Dip the activated gold electrode into 10μM cDNA (5'-CTA AGT AAC TCT GCA CTC TTA TAT ATCATA GAA TTG GTA GAT-(CH 2 )6-SH-3') in PBS solution (50mM, pH 7.4), incubated at 36°C for 3h to form a cDNA-modified Au electrode (cDNA-Au).
[0088] After washing with wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com