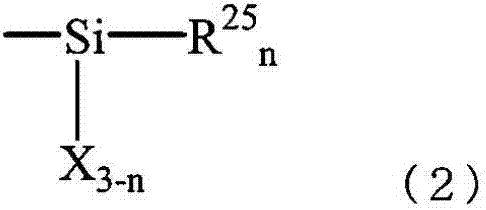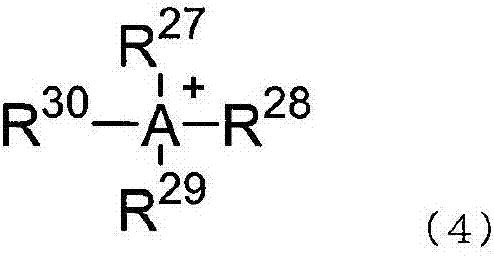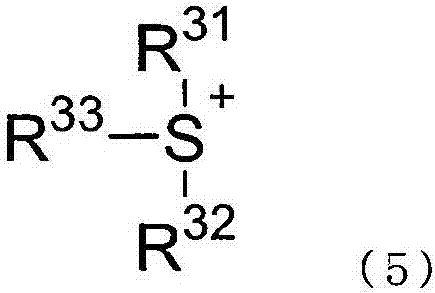Two-part curable composition
A curable composition, two-component technology, applied in the direction of non-polymer organic compound adhesives, adhesives, etc., can solve the problem of no flexibility of the cured product, poor resistance to cold and heat cycles, damage to the flexibility of the adherend, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0167] The present invention will be explained more specifically based on examples, but the present invention is not limited to these. In addition, in the following, part and% are the basis of mass unless otherwise specified.
[0168] 1. Evaluation method
[0169] (1) Molecular weight
[0170] Device: Alliance 2695 (manufactured by Waters)
[0171] Column: TSKgel SuperMultiporeHZ-H, 2; TSKgel SuperHZ-2500, 2 connected (manufactured by Tosoh Corporation)
[0172] Column temperature: 40℃
[0173] Eluent: tetrahydrofuran 0.35ml / min
[0174] Detector: RI
[0175] The molecular weight measured by GPC is converted based on the molecular weight of polystyrene.
[0176] (2) Bonding speed
[0177] According to JIS K6861 "Test Method for α-Cyanoacrylate Adhesives", the bonding speed was measured in an environment of 23° C. and 60% RH. The test pieces used are as follows.
[0178] Test piece: ABS resin made by Ube Secon Corporation, trade name "GSE"
[0179] (3) Resistance to cold and heat cycles
[018...
Synthetic example 1
[0192] Synthesis Example 1 (Methyl tri-n-octyl ammonium trifluoromethanesulfonate)
[0193] A 50ml eggplant-shaped flask was charged with 4.041 g (10.00 mmol) of methyl tri-n-octyl ammonium chloride (reagent), anion exchange resin (manufactured by Orgino, trade name "Anbillite IRA900A OH AG", strong base type) 13.2 g (20 mg equivalent), 25 ml of toluene, and stirring at room temperature for 48 hours. After filtering out the ion exchange resin, 1.501 g (10.00 mmol) of trifluoromethanesulfonic acid was dropped under ice cooling. The ice bath was removed, and stirring was continued for another 12 hours at room temperature. It was washed 3 times with 25 ml of ion-exchanged water, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The obtained residue was dissolved in 25 ml of methanol, and the insoluble matter was filtered out. The solvent was distilled off under reduced pressure to obtain 5.022 g of a pale yellow semi-solid ( Salt A).
Synthetic example 2
[0194] Synthesis Example 2 (4-methyl-1-octylpyridine bromide )
[0195] A 30 ml eggplant-shaped flask was charged with 9.313 g (0.100 mol) of 4-picoline (reagent), 19.312 g (0.100 mol) of octyl bromide (reagent), and 10 ml of acetonitrile, and stirred at 80°C for 24 hours. The solvent was distilled off under reduced pressure and dried to obtain 4-methyl-1-octylpyridine bromide as a colorless liquid 26.311g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com