Peptide having osteoclast differentiation and activation inhibition, and use of same
An activity-inhibiting, osteoclast technology, used in medical preparations containing active ingredients, peptides, bone diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0046] Synthesis Example 1: Peptide Synthesis
[0047] 700 mg of Chloro trityl chloride resin (Chloro trityl chloride resin; CTL resin, Novabiochem Cat No. 01-64-0021) was put into the reaction vessel, and 10 ml of dichloromethane (MC) was added to stir for 3 minutes . The solution was removed and 10 ml of dimethylformamide (DMF) was added to stir for 3 minutes, and the solvent was removed again. The dichloromethane (DCM) solution of 10ml is put into reactor, and put into the Fmoc-Arg (Pbf)-OH (Swiss Bachem (Bachem, Swiss)) of 200mmol and the diisopropylethylamine of 400mmol ( DIEA), stirred and dissolved uniformly, stirred for 1 hour and reacted. Wash after the reaction, dissolve methanol and diisopropylethylamine (2:1) in dichloromethane, react for 10 minutes, and wash with excess dichloromethane / dimethylformamide (1:1). The solution was removed and 10 ml of dimethylformamide was added to stir for 3 minutes, and the solvent was removed again. 10 ml of a deprotection solu...
Embodiment 1
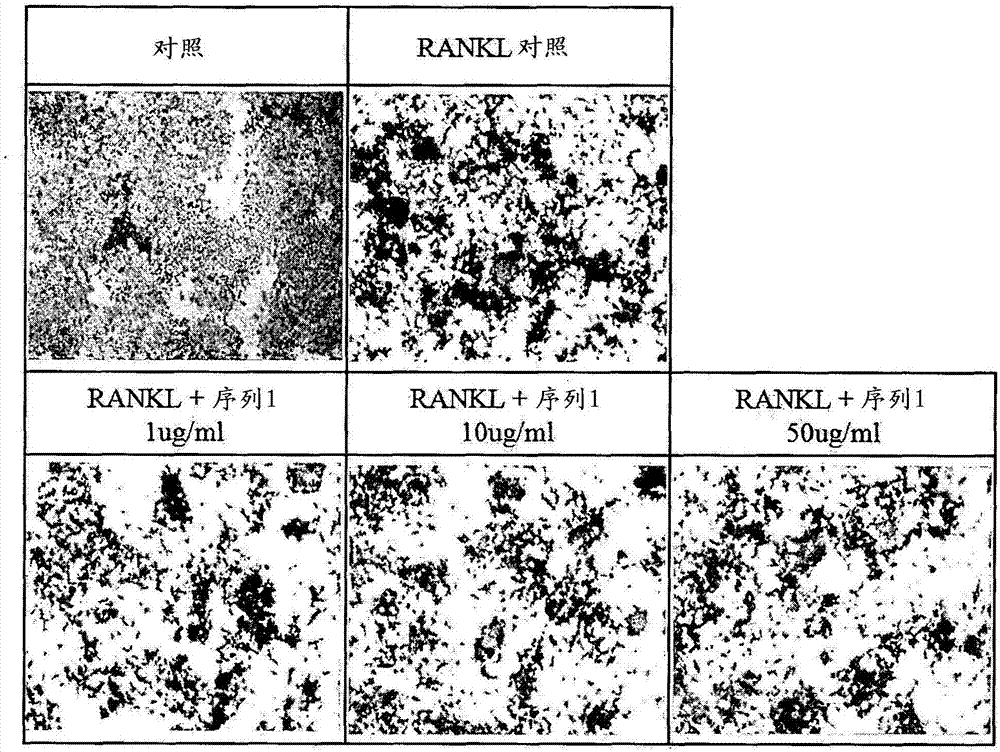
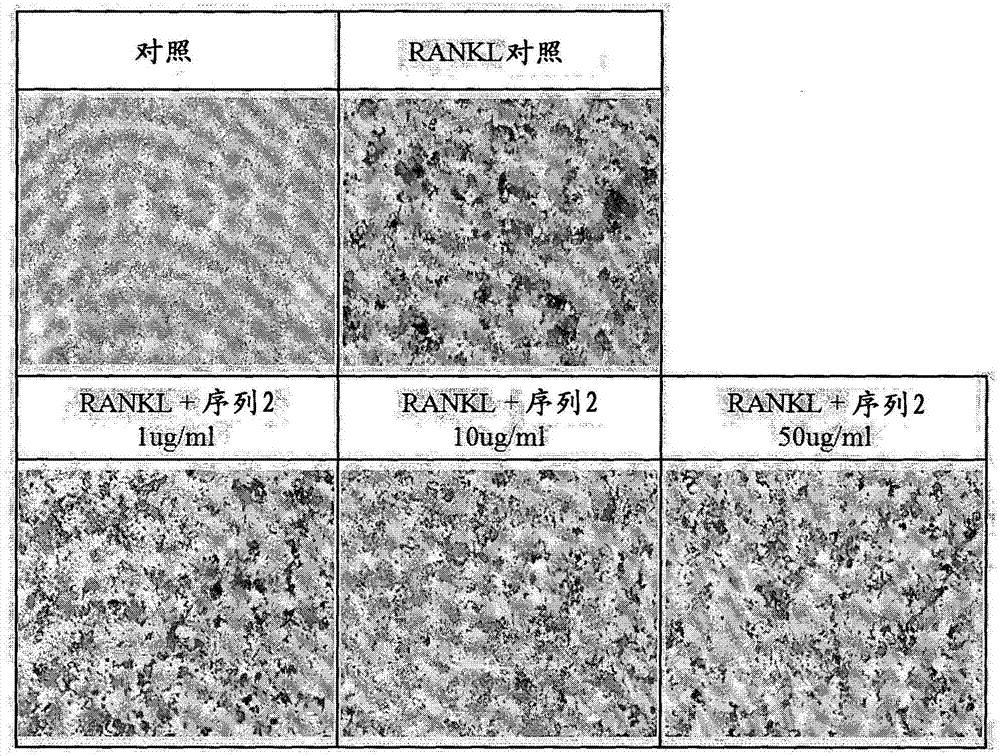
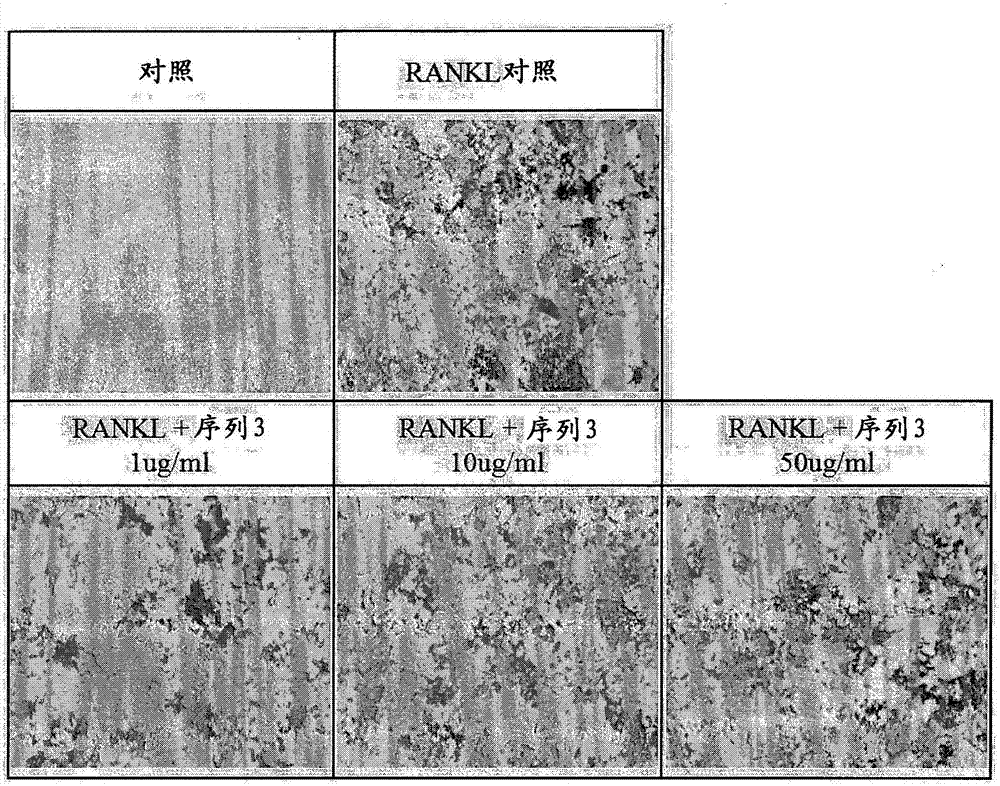
[0053] Example 1: Tartrate-resistant acid phosphatase staining
[0054] The degree of tartrate-resistant acid phosphatase protein expressed when osteoclasts were differentiated was confirmed by staining, and the tendency to decrease when the peptide was treated was observed.
[0055] Take 1×10 4 After seeding Raw264.7 macrophages in a 48-well plate, the cells / well were simultaneously treated with peptides (independent peptides or 50 ng / ml NF-KB receptor activator ligand simultaneously) by concentration, and Differentiation was induced by culturing for 5 days. Staining for tartrate-resistant acid phosphatase was performed using the acid phosphatase kit from Sigma aldrich. After adding the fixation buffer, the reaction was performed for 30 seconds, followed by washing with distilled water. 200 µl of staining solution was placed in each well, reacted at 37° C. for 30 minutes, and washed with distilled water. Observation was carried out with a microscope after drying for one d...
Embodiment 2
[0057] Example 2: Reverse transcription-polymerase chain reaction related to tartrate-resistant acid phosphatase and cathepsin K (cathepsin K)
[0058] The mRNA levels of tartrate-resistant acid phosphatase and cathepsin K (mRNA level) expressed when osteoclasts were differentiated were confirmed by reverse transcription-polymerase chain reaction, and observed when peptides were treated tendency to decrease.
[0059] Take 1×10 4 Cell Density of Cells / well RAW 264.7 macrophages were seeded in 48-well plates. At the same time, 50 ng / ml of nuclear factor KB receptor activator ligand and peptide were treated at concentrations (10 μg / ml, 50 μg / ml), and cultured in a 37° C. incubator for 5 days. After recovering the cultured cells, RNA was prepared by treating RNA extraction solution (Easy Blue, Intron) and then synthesized using antisense premix (RTpremix) (Intron) Complementary deoxyribonucleic acid (cDNA). Polymerase chain reaction was performed by using primers for the respe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com