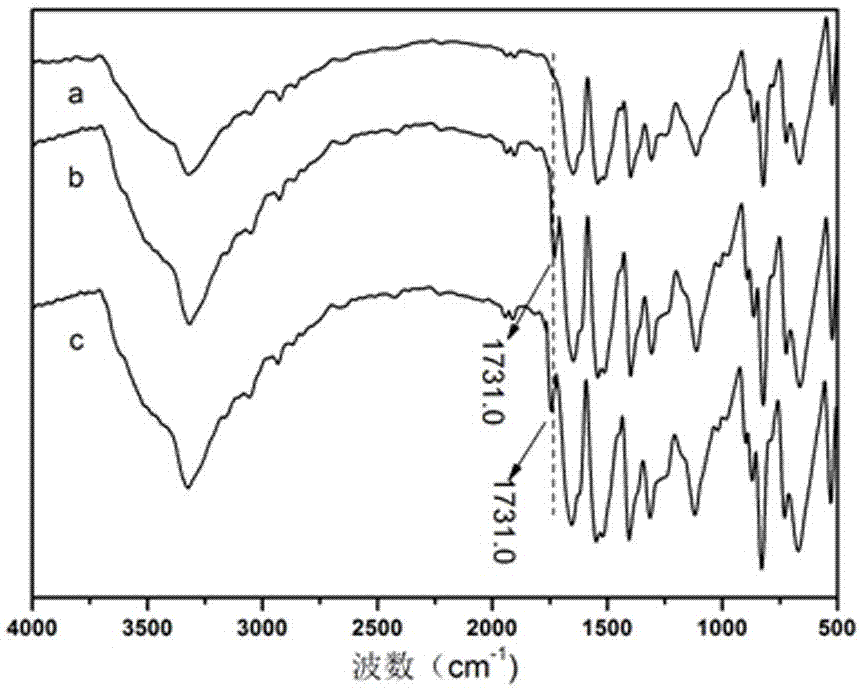
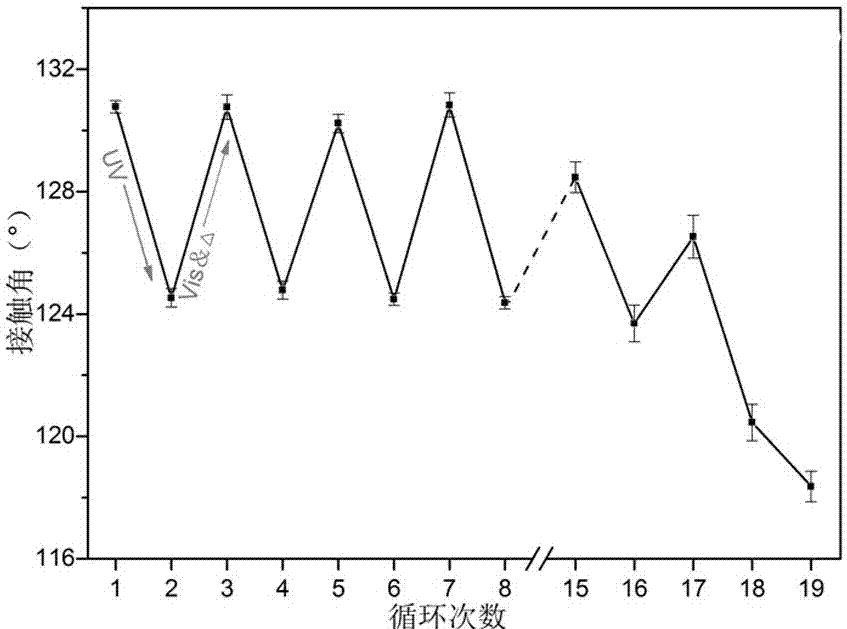
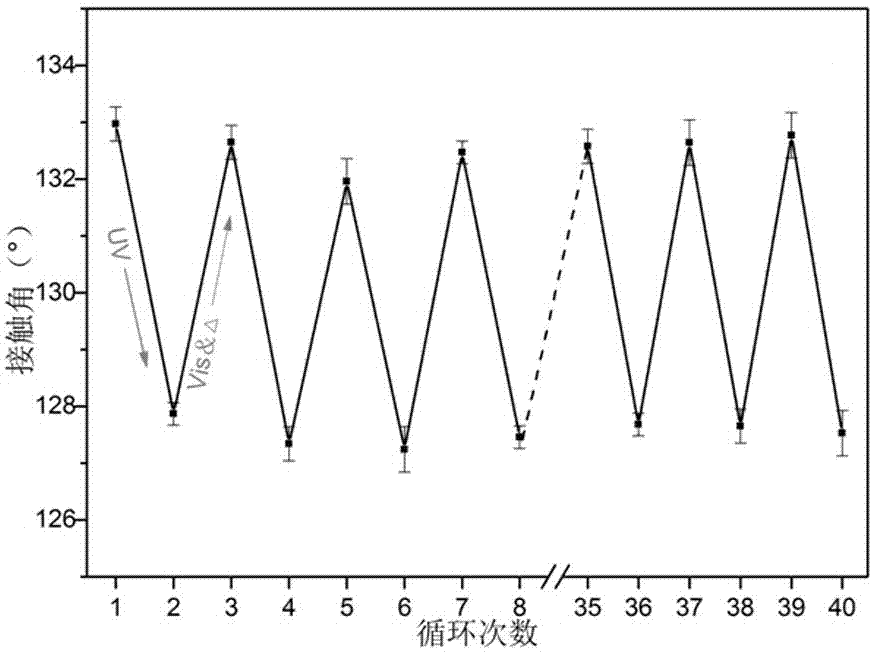
Intelligent textile finishing agent with convertible surface hydrophilicity and hydrophobicity and method for preparing intelligent textile finishing agent
A fabric finishing agent, intelligent technology, applied in the preparation of amino compounds from amines, fiber types, fiber treatment, etc., can solve the problems of poor thermal stability and low ground state energy level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] (1) p -Nafluorobutylaniline
[0084] Add 3.44 g of p-bromoaniline, 5 g of copper powder (catalyst) and 100 ml of dimethyl sulfoxide (DMSO) into a 250 ml three-necked flask equipped with a magnetic stirring bar, a thermometer and a condenser, and heat to 60 °C with stirring. Then 9 g of nonafluoroiodobutane was dissolved in 25 ml of DMSO and added to a constant pressure dropping funnel, and slowly dropped into a three-necked flask. After the nonafluoroiodobutane solution was added dropwise, the reaction system was heated to 120 °C and refluxed for 24 h. The reaction system was cooled to room temperature, and the reaction was poured into a 500 ml beaker, and 100 ml of deionized water and 200 ml of anhydrous ether were added at the same time, the layers were stirred, and the copper powder was filtered off. Pour the filtrate into a 500 ml separatory funnel to separate the organic layer and wash it with deionized water (30 ml x 3 times), dry over anhydrous magnesium sulfat...
Embodiment 2
[0099] (1) p -Synthesis of Tridecafluorohexylaniline
[0100] Add 3.44 g of p-bromoaniline, 5 g of copper powder (catalyst) and 100 ml of dimethyl sulfoxide (DMSO) into a 250 ml three-neck flask equipped with a magnetic stirrer, a thermometer and a condenser tube, stir and heat to 60 °C. Then 11.6 g trifluoroiodohexane was dissolved in 25 ml of DMSO and added to a constant pressure dropping funnel, and slowly dropped into a three-necked flask. After the dropwise addition of tridecafluoroiodohexyl solution was completed, the reaction system was heated to 120 °C and refluxed for 24 h. The reaction system was cooled to room temperature, the reaction was poured into a 500 ml beaker, 100 ml of deionized water and 200 ml of anhydrous ether were added at the same time, the layers were stirred, and the copper powder was filtered off. Pour the filtrate into a 500 ml separatory funnel to separate the organic layer and wash it with deionized water (30 ml x 3 times), dry over anhydrous ...
Embodiment 3
[0113] (1) p -Heptadecafluorooctylaniline
[0114] Add 3.45 g of p-bromoaniline, 5.15 g of copper powder (catalyst) and 100 ml of dimethyl sulfoxide into a 250 ml three-neck flask equipped with a magnetic stirring bar, a thermometer and a condenser, and heat to 60 °C with stirring. Then 9.21 g of heptadecafluoroiodoctane was dissolved in 25 ml of dimethyl sulfoxide and added to a constant pressure dropping funnel, and slowly dropped into a three-necked flask. After the nonafluoroiodobutane solution was added dropwise, the temperature of the reaction system was raised to 115° C. and refluxed for 12 hours. The reaction system was cooled to room temperature, and the reaction was poured into a 500 ml beaker, 100 ml of deionized water and 200 ml of anhydrous ether were added at the same time, the layers were stirred, and the copper powder was filtered off. Pour the filtrate into a 500 ml separatory funnel to separate the organic layer and wash with deionized water (30 ml × 3 time...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com