Method for synthesizing <18>F ethyl choline
A technology of ethylcholine and dimethylethanolamine, which is applied in the direction of organic chemical methods, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of lack of versatility, and achieve simple production equipment and processes and high reaction yields The effect of high and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
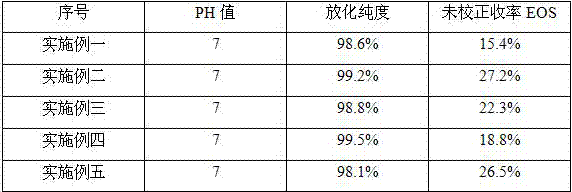
Embodiment 1
[0029] A 2-bromotrifluoromethanesulfonic acid ethyl ester (BrC 2 h 4 OTf) was synthesized as a starting material 18 F ethylcholine ( 18 F-FECH) method.
[0030] (1) Continuous bombardment of H with a cyclotron proton beam 2 18 O, production 18 f - , with helium 18 f - Transfer from the target to the target water recovery bottle of the synthesis module.
[0031] (2) Press out from the target water recovery bottle 18 f - Through the QMA column, 18 f - Captured on the QMA column.
[0032] (3) with 0.9ml K 2.2.2 / K 2 CO 3 The solution will be adsorbed on the QMA column 18 f - Rinse to the reaction tube, and then start heating the reaction tube to remove the water and acetonitrile in the system. After evaporating to dryness, continue to add 0.5 ml of anhydrous acetonitrile to the reaction flask, and heat and evaporate to dryness again.
[0033] (4) Add BrC dissolved in o-dichlorobenzene 2 h 4 OTf (10 μl BrC 2 h 4 OTf is dissolved in 1.0ml o-dichlorobenzene...
Embodiment 2
[0037] A 2-bromotrifluoromethanesulfonic acid ethyl ester (BrC 2 h 4 OTf) was synthesized as a starting material 18 F ethylcholine ( 18 F-FECH) method.
[0038] (1) Continuous bombardment of H with a cyclotron proton beam 2 18 O, production 18 f - , with helium 18 f - Transfer from the target to the target water recovery bottle of the synthesis module.
[0039] (2) Press out from the target water recovery bottle 18 f - Through the QMA column, 18 f - Captured on the QMA column.
[0040] (3) with 0.9ml K 2.2.2 / K 2 CO 3 The solution will be adsorbed on the QMA column 18 f - Rinse to the reaction tube, and then start heating the reaction tube to remove the water and acetonitrile in the system. After evaporating to dryness, continue to add 0.5 ml of anhydrous acetonitrile to the reaction flask, and heat and evaporate to dryness again.
[0041] (4) Add BrC dissolved in o-dichlorobenzene 2 h 4 OTf (15 μl BrC 2 h 4 OTf is dissolved in 1.0ml o-dichlorobenzene...
Embodiment 3
[0045] A 2-bromotrifluoromethanesulfonic acid ethyl ester (BrC 2 h 4 OTf) was synthesized as a starting material 18 F ethylcholine ( 18 F-FECH) method.
[0046] (1) Continuous bombardment of H with a cyclotron proton beam 2 18 O, production 18 f - , with helium 18 f - Transfer from the target to the target water recovery bottle of the synthesis module.
[0047] (2) Press out from the target water recovery bottle 18 f - Through the QMA column, 18 f - Captured on the QMA column.
[0048] (3) with 0.9ml K 2.2.2 / K 2 CO 3 The solution will be adsorbed on the QMA column 18 f - Rinse to the reaction tube, and then start heating the reaction tube to remove the water and acetonitrile in the system. After evaporating to dryness, continue to add 0.5 ml of anhydrous acetonitrile to the reaction flask, and heat and evaporate to dryness again.
[0049] (4) Add BrC dissolved in o-dichlorobenzene 2 h 4 OTf (20 μl BrC 2 h 4 OTf is dissolved in 1.0ml o-dichlorobenzene...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com