Oxadiazole-containing imide blue-ray iridium complex as well as preparation method thereof and application thereof
A technology of oxadiazole imide and iridium complex, which is applied in the direction of indium organic compounds, platinum group organic compounds, chemical instruments and methods, etc., can solve the problems of device efficiency roll-off, limit the application of OLEDs, and unsatisfactory performance. Achieve the effects of improving efficiency roll-off, improving carrier transport performance, and improving luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment one: the synthesis of complex 1:
[0034] Under anhydrous and oxygen-free conditions, bis-[2-(2,4-difluorophenyl)pyridine]iridium dichloride (1.07 g, 0.88 mmol) and 2-(N-benzoyl)- The potassium salt of amino-5-phenyl-[1,3,4]-oxadiazole (0.65 g, 2.16 mmol) was dissolved in 20 ml of ethylene glycol monoethyl ether, reacted at 130°C for 12 hours, filtered, and finally Purified by column chromatography to obtain the complex bis[2-(2,4-difluorophenyl)pyridine]-{2-(N-benzoyl)-amino-5-phenyl-[1,3,4 ]-oxadiazole} iridium (1). The yield is 33%:
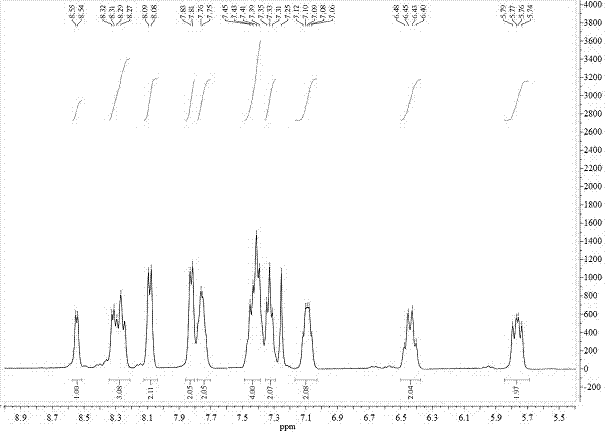
[0035] 1 H NMR (400MHz, CDCl 3 ,δ):8.55(d,J=4.0Hz,1H),8.32–8.25(m,3H),8.08(d,J=4.0Hz,2H),7.82(d,J=8.0Hz,2H),7.76 (d,J=4.0Hz,2H),7.45–7.39(m,4H),7.33(t,J=8.0Hz,,2H),7.12–7.06(m,2H),6.48–6.40(m,2H) ,5.79–5.74(m,2H).
[0036] MS(ESI-MS)[m / z]:m / z 837.75(M+H) + .
[0037] Elemental analysis results: Calculated values: C(%): 53.11, H(%): 2.65, N(%): 8.37.
[0038] Measured values: C (%): 53.16, H (%): 2.68, N (%): 8.33. ...
Embodiment 2
[0040] Under anhydrous and oxygen-free conditions, bis-[2-(2,4-difluorophenyl)-4-methylpyridine] iridium dichloride (1.08 g, 0.88 mmol) and 2-(N- Diphenylphosphinyl)-amino-5-phenyl-[1,3,4]-oxadiazole potassium salt (0.86 g, 2.16 mmol) was dissolved in 15 ml of ethylene glycol monoethyl ether at 130 ℃ for 12 hours, filtered, and finally purified by column chromatography to obtain the complex bis[2-(2,4-difluorophenyl)-4-methylpyridine]-{2-(N-diphenylphosphine Acyl)-amino-5-phenyl-[1,3,4]-oxadiazole} iridium (2).
[0041] The yield is 38%:
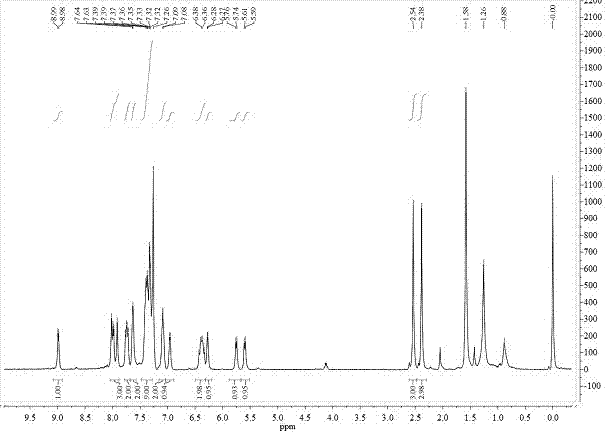
[0042] 1 H NMR (400MHz, CDCl 3 ,δ):8.99(d,J=4.0Hz,1H),8.02–7.92(m,3H),7.76–7.72(m,2H),7.63(s,2H),7.39–7.32(m,9H), 7.09(d,J=4.0Hz,2H),6.97(d,J=4.0Hz,1H),6.43–6.33(m,2H),6.28(d,J=4.0Hz,1H),5.76(d,J =8.0Hz, 1H), 5.61(d, J=8.0Hz, 1H), 2.54(s, 3H), 2.38(s, 3H).
[0043] MS(ESI-MS)[m / z]:m / z 961.75(M+H) + .
[0044] Elemental analysis results: Calculated values: C(%): 55.00, H(%): 3.25, N(%): 7.29.
[0045] Measured values: C (%): 54.98, H...
Embodiment 3
[0047] Under anhydrous and oxygen-free conditions, bis-[2′,6′-difluoro-2,3′-bipyridine] iridium dichloride (1.07 g, 0.88 mmol) and 2-(N-diphenyl Phosphinyl)-amino-5-phenyl-[1,3,4]-oxadiazole potassium salt (0.86 g, 2.16 mmol) was dissolved in 15 ml of ethylene glycol monoethyl ether and reacted at 130°C 12 hours, filtered, and finally purified by column chromatography to obtain the complex bis[2′,6′-difluoro-2,3′-bipyridine]-{2-(N-diphenylphosphoryl)-amino -5-Phenyl-[1,3,4]-oxadiazole}iridium (3). The yield is 44%:
[0048] 1 H NMR (400MHz, DMSO-d 6 ,δ):9.03(d,J=4.0Hz,1H),8.34(d,J=4.0Hz,1H),8.24(d,J=8.0Hz,1H),8.11(d,J=8.0Hz,2H ),7.84(dd,J=8.0Hz and 4.0Hz,1H),7.76–7.71(m,2H),7.55–7.46(m,9H),7.35(t,J=4.0Hz,1H),7.28–7.18 (m, 4H), 6.94 (dd, J = 8.0Hz and 4.0Hz, 1H), 5.72 (s, 1H), 5.52 (s, 1H).
[0049] MS(ESI-MS)[m / z]:m / z 935.46(M+H) + .
[0050] Elemental analysis results: Calculated values: C(%): 51.39, H(%): 2.70, N(%): 10.49.
[0051] Measured values: C (%): 51.44, H ...
PUM
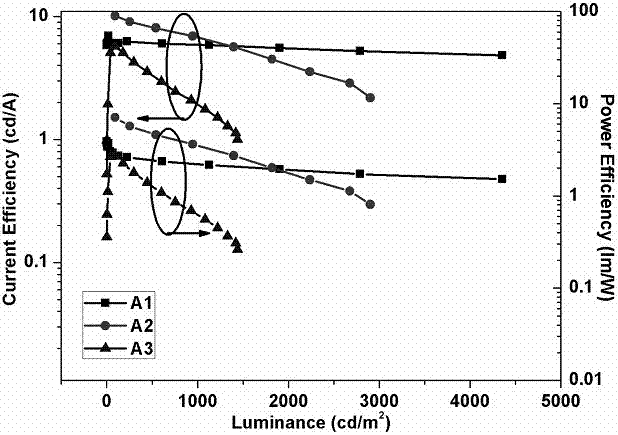
| Property | Measurement | Unit |
|---|---|---|
| external quantum efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com