A polymer donor material, its preparation method and organic solar cell comprising it
A technology of solar cells and polymers, applied in the field of solar cells, can solve problems affecting the photoelectric performance of cells, poor polymer solubility, and material film-forming properties, so as to promote π-π stacking, ensure planarity and solubility , the effect of lowering the energy level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0055] This preparation example provides a polymer donor material P1 Its synthetic route is as follows:
[0056]
[0057] The specific synthesis steps are as follows:
[0058] (1) Difluorodibromodihydroxybenzene raw material 1 (207 mg), 1-bromo-octane (1.05 g), sodium hydride (326 mg), and anhydrous DMF (2 mL) were sequentially added to a 25 mL Schlenck tube, under nitrogen protection, React at 130°C for 24 hours, add 3 mL of saturated aqueous sodium chloride solution, extract three times with petroleum ether, evaporate the organic layer under reduced pressure, and then pass through a silica gel column, using dichloromethane: petroleum ether (volume ratio 1:8) as the eluent, The product 2 was obtained as a colorless oil (348 mg, 66% yield).
[0059] Characterization of Compound 2:
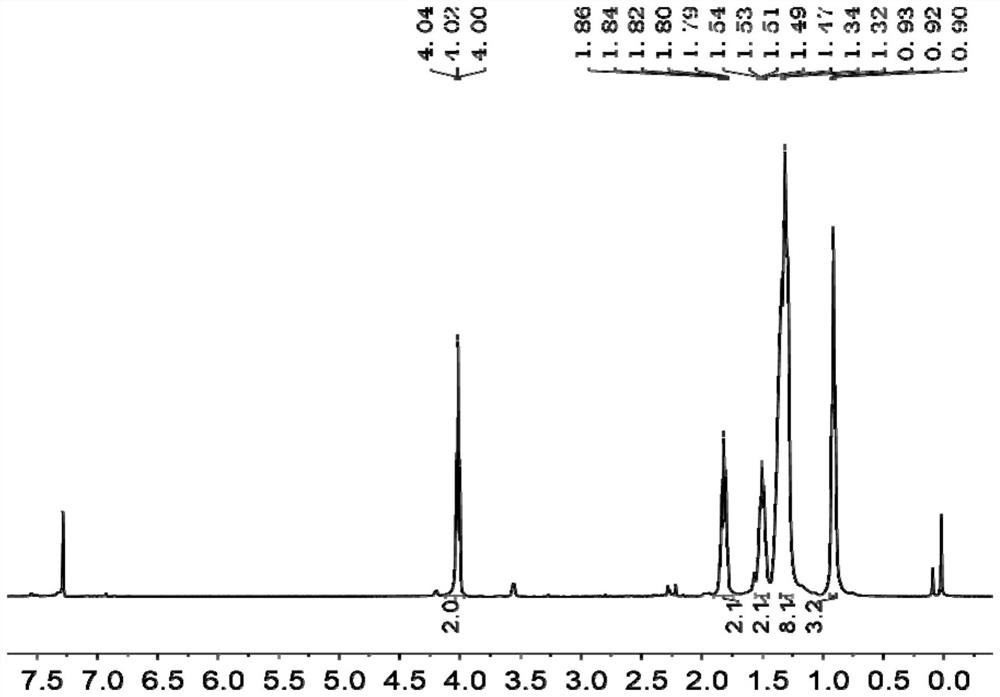
[0060] 1 H NMR (CDCl 3 ,400MHz,δ / ppm):4.02(t,J=6.7Hz,2H),1.82(m,2H),1.51(m,2H),1.33(m,8H),0.92(t,J=6.3Hz, 3H). The H NMR spectrum of compound 2 is as follows figure 1 shown.
[0061] ...
preparation example 2
[0079] This preparation example provides a polymer donor material P2 Its synthetic route is as follows:
[0080]
[0081] The specific synthesis steps are as follows:
[0082] Steps (1), (2), (3) are the same as in Preparation Example 1;
[0083] (4) Compound 4 (47 mg), compound 6 (62 mg) and Pd (PPh) were added to a 10 mL Schlenck tube in sequence 3 ) 4 (4.7mg) and toluene (2mL), reacted under nitrogen protection at 110 ° C for 24 hours, cooled to room temperature, the reaction solution was added dropwise to 150mL of methanol for chromatography to obtain a crude product, which was then used respectively with n-hexane, dichloromethane, Chloroform was subjected to Soxhlet extraction, the obtained chloroform solution was collected and gradually dropped into methanol, and a red solid product was obtained by chromatography, which was the polymer donor material P2 (53 mg, yield 68%).
[0084] Characterization of polymer donor material P2:
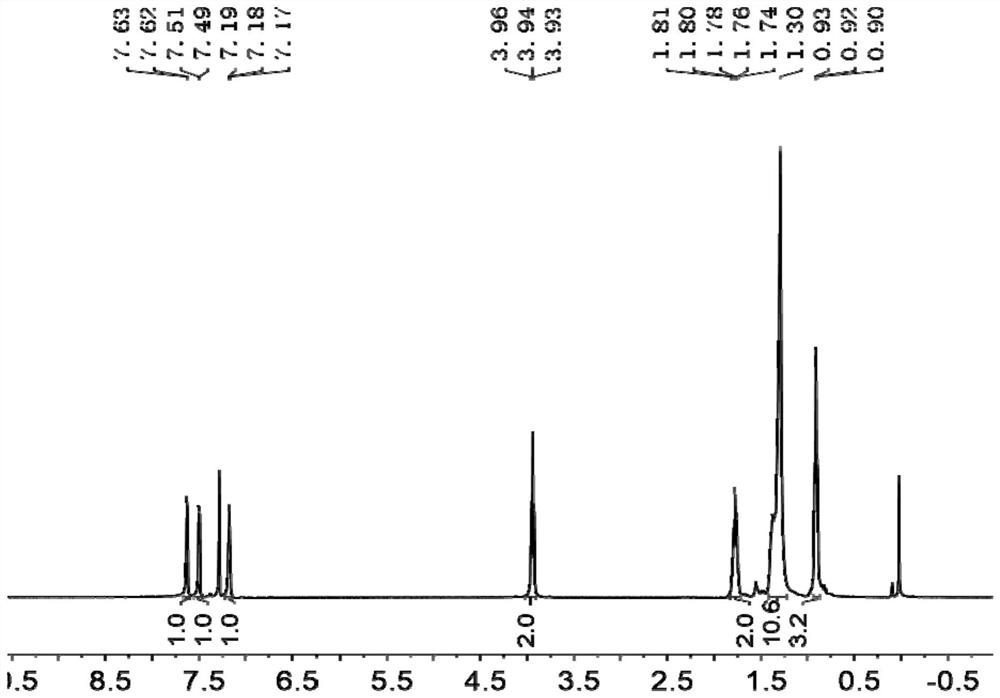
[0085] 1 H NMR (CDCl 3 , 400MH...
preparation example 3
[0089] This preparation example provides a polymer donor material P3 Its synthetic route is as follows:
[0090]
[0091] The specific synthesis steps are as follows:
[0092] (1) Into a 25mL Schlenck tube, add raw material 7 (207mg), 1-bromo-octane (1.05g), sodium hydride (326mg), anhydrous DMF (2mL) in sequence, under nitrogen protection, react at 130°C for 24 hours, add 3 mL of saturated aqueous sodium chloride solution was extracted three times with petroleum ether, the organic layer was evaporated under reduced pressure and passed through a silica gel column, and dichloromethane: petroleum ether (volume ratio 1:8) was the eluent to obtain a colorless oily product 8 ( 380 mg, 72% yield).
[0093] Characterization of Compound 8:
[0094] Mass spectral data: MALDI-TOF MS (m / z): 528.1 (M + ).
[0095] (2) Compound 8 (150mg), 2-thiophenetrimethyltin reagent (277mg) and Pd(PPh) were added to a 25mL Schlenck tube in sequence 3 ) 4 (32.4 mg), anhydrous DMF (3 mL), reac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com