Full-human-derived anti-CD19 monoclonal antibody and production method thereof
A monoclonal antibody, fully human technology, used in biochemical equipment and methods, chemical instruments and methods, and botanical equipment and methods, etc., can solve the problems of reduced antibody efficacy and side reactions, and achieve good stability and safety. The effect of low sex and immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
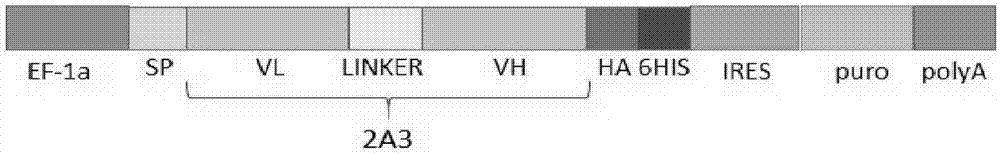
[0026] Example 1: Sequence and vector construction of CD19 single-chain antibody 2A3
[0027] The sequence of the CD19 single-chain antibody 2A3 in the present invention is formed by connecting VL and VH through a linker sequence, and the structure is VL-Linker-VH. The amino acid sequence of its linker is GGGGSGGGGSGGGGS.
[0028] The sequence characteristic of VL is that it contains three light chain complementarity determining regions CDR L1, CDR L2 and CDR L3 shown in Table 1. See the sequence listing for the complete sequence of VL. The sequence is derived from a human germline gene
[0029] The sequence of VH is characterized by containing three heavy chain complementarity determining regions VH CDR1, CDR2, and CDR3 shown in Table 1. The complete sequence of VH is shown in the sequence listing. The sequence is derived from a human germline gene
[0030] The complete 2A3 sequence consists of VL, LINKER, and VH in sequence, as shown in the sequence listing. It should ...
Embodiment 2
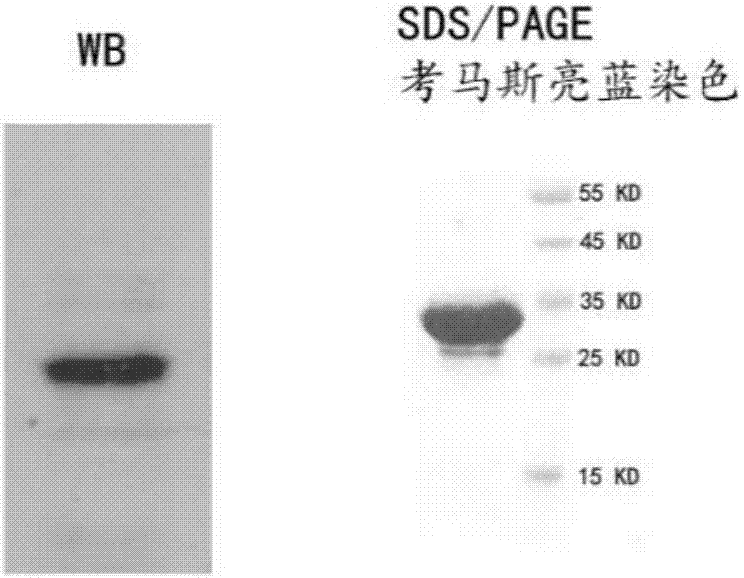
[0037] Example 2: Preparation of CD19 single-chain antibody
[0038] Transfection of HEK293T cells with recombinant plasmid pEF-2A3: HEK293T cells were inoculated into T175 flasks one day before transfection, and transfected the next day when the cell density was about 80%. First, take 1.5ml of serum-free culture and add 60ug of plasmid; then take 1.5ml of serum-free medium and add 120ul Lipofectamine2000 liposome transfection reagent; mix the plasmid and liposome solution evenly, let stand at room temperature for 15 minutes, and then add to cell culture After 6 hours, the medium was replaced with fresh DMEM+10% FBS medium; after 72 hours, the culture supernatant was harvested. The supernatant contains the single-chain antibody.
[0039] Purification of single-chain antibody 2A3: first add 500ul of nickel filler suspension to the gravity column, and add 10ml of PBS equilibrium system to it after the liquid is drained; add the medium supernatant to the gravity column and let i...
Embodiment 3
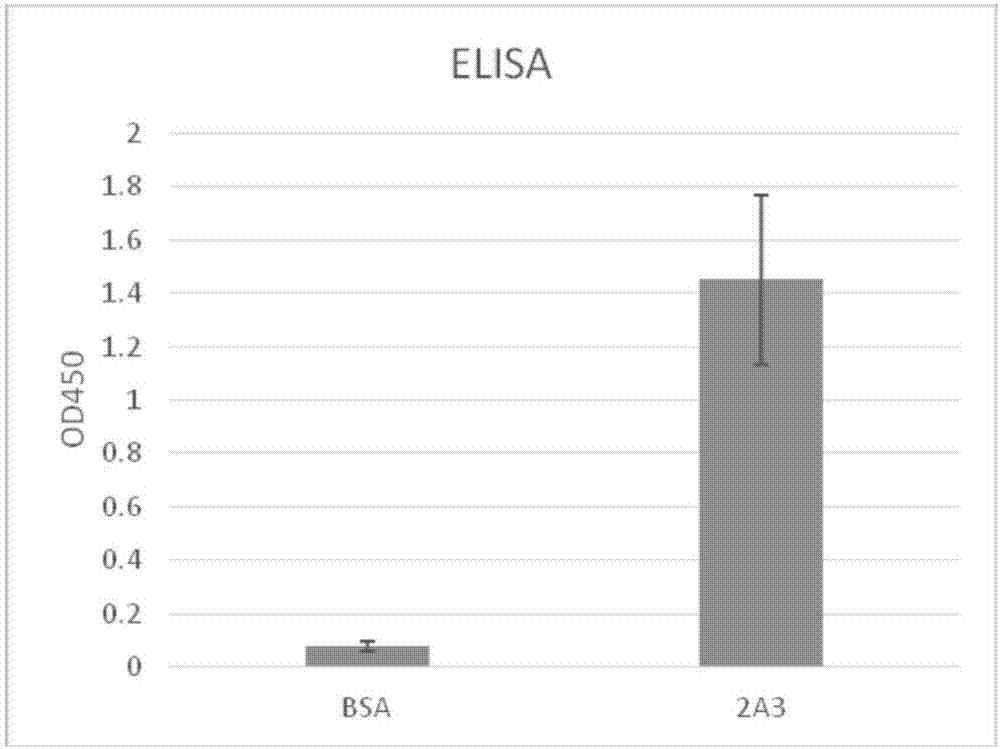
[0041] Example 3: Detection of CD19 Antigen Binding Ability
[0042] The binding ability of CD19 single chain antibody 2A3 to CD19 antigen was detected by ELISA method. Firstly, the CD19 antigen was used to coat the microtiter plate, the antigen concentration was 2ug / ml, the coating solution was sodium carbonate buffer, and the coating was performed overnight at 4°C. Block the microtiter plate and single-chain antibody 2A3 with 2% MPBS for 2 hours at room temperature. Add 100ul of blocked 2A3 to the blocked ELISA plate, add BSA to the control well as a control, and incubate at room temperature for 1 hour. Wash the plate 5 times with PBST and 2 times with PBS; add anti-HA-HRP antibody and incubate for 1 hour; wash the plate 5 times with PBST and 2 times with PBS; add TMB chromogenic solution, 100 μl / well; room temperature The color was developed for 5-30 minutes, until the well showed an appropriate degree of blue, and 50ul of concentrated sulfuric acid was added to terminate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com