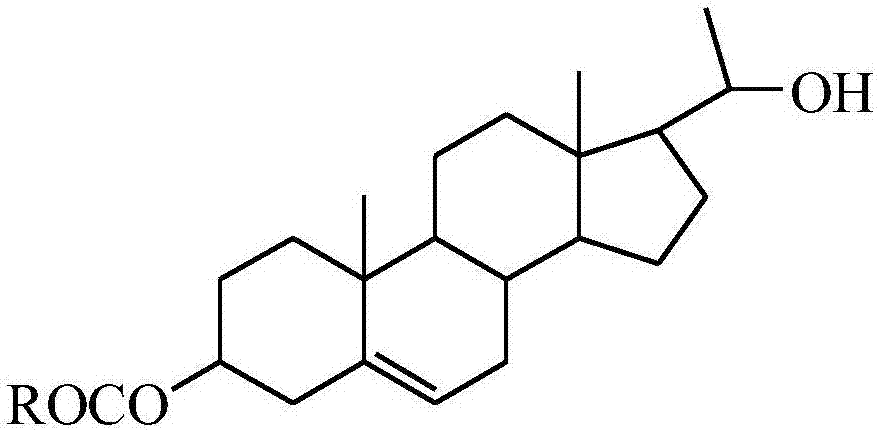
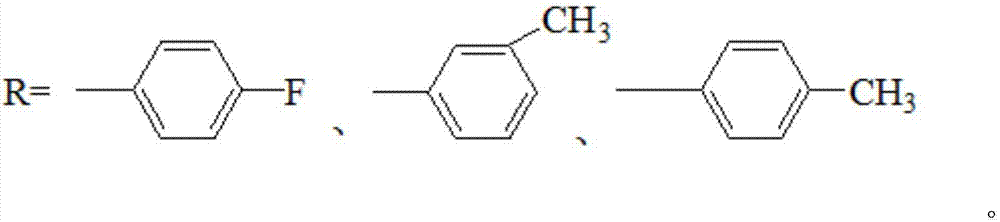
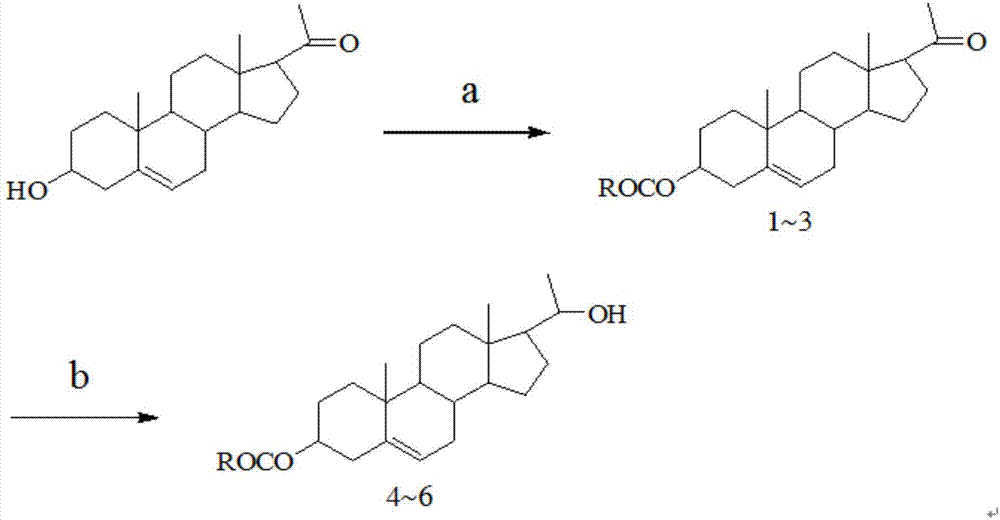
20-hydroxy-pregnen-3-aryl ester base pregnene compound, synthetic method thereof and application thereof in preparation of anti-tumor drugs
A synthesis method and compound technology, which can be used in antitumor drugs, steroids, drug combinations, etc., and can solve problems such as applications that have not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of 20-hydroxyl-pregnene-3-p-fluorobenzoate (4)
[0028] Step 1: the preparation of pregnene-3-p-fluorobenzoate (1)
[0029] Weigh 0.360g (about 1 mmol) of pregnenolone, put it in a 100mL eggplant-shaped flask, pipette 10mL of pyridine into the flask, stir the pregnenolone until it is completely dissolved, and then add 400uL of p-fluorobenzoyl chloride. React in an oil bath at 40°C, track the reaction with TLC, stop the reaction when no raw material is reached, and react for 24 hours. When processing the reaction product, first add 15mL of ice water, extract the aqueous phase with ethyl acetate (15mL×3), combine the organic phases, wash with 1mol / L dilute hydrochloric acid, and then extract with saturated NaCl solution (15mL×3). Wash the organic phase, followed by anhydrous Na 2 SO 4 dry. Finally, the organic solvent is distilled off under reduced pressure, and column chromatography separates (eluent: V 乙酸乙酯 :V 石油醚 =1:5), 0.614g of white solid was obta...
Embodiment 2
[0032] Preparation of 20-hydroxyl-pregnene-3-m-methylbenzoate (5)
[0033] Step 1: the preparation of pregnene-3-m-methylbenzoate (2)
[0034] Weigh 0.339g (about 1 mmol) of pregnenolone, place it in a 100mL eggplant-shaped flask, pipette 10mL of pyridine into the flask, stir the pregnenolone until it is completely dissolved, and then transfer 400uL m-toluoyl chloride. React in an oil bath at 40°C, track the reaction with TLC, stop the reaction when no raw material is reached, and react for 24 hours. When processing the reaction product, first add 15mL of ice water, extract the aqueous phase with ethyl acetate (15mL×3), combine the organic phases, wash with 1mol / L dilute hydrochloric acid, and then extract with saturated NaCl solution (15mL×3). Wash the organic phase, followed by anhydrous Na 2 SO 4 dry. Finally, the organic solvent is distilled off under reduced pressure, and column chromatography separates (eluent: V 乙酸乙酯 :V 石油醚 =1:5), 0.519g of white solid was obtain...
Embodiment 3
[0037] 20-hydroxyl-pregnene-3-p-methylbenzoate (6)
[0038] Step 1: the preparation of pregnene-3-p-methylbenzoate (3)
[0039] Weigh 1.558g (about 5mmol) of pregnenolone, put it in a 100mL eggplant-shaped flask, pipette 15mL of pyridine into the flask, stir the pregnenolone until it is completely dissolved, and then add 400uL of p-toluoyl chloride. React in an oil bath at 40°C, track the reaction with TLC, stop the reaction when no raw material is reached, and react for 24 hours. When processing the reaction product, first add 15mL of ice water, extract the aqueous phase with ethyl acetate (15mL×3), combine the organic phases, wash with 1mol / L dilute hydrochloric acid, and then extract with saturated NaCl solution (15mL×3). Wash the organic phase, followed by anhydrous Na 2 SO 4 dry. Finally, the organic solvent is distilled off under reduced pressure, and column chromatography separates (eluent: V 乙酸乙酯 :V 石油醚 =1:5), 1.962g of white solid was obtained, the yield was: 9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com