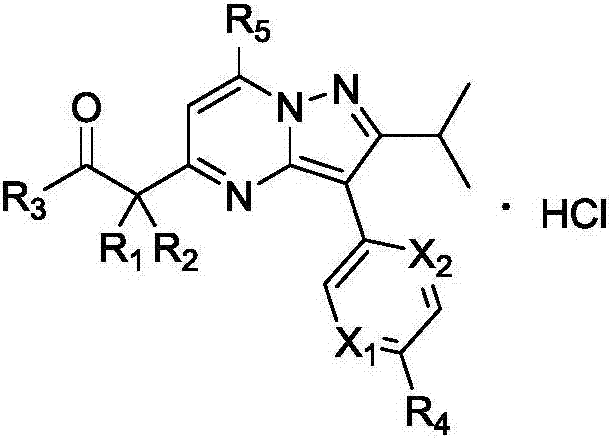
TRPC6 inhibitor with anti-gastric cancer activity, and preparation method and application thereof
A technology of use and alkyl, applied in the field of pyrazolo[1,5-a]pyrimidine derivatives and their treatment of gastric cancer, can solve the problems of inability to find activity and oral availability, low oral availability, and high in vivo clearance rate , to achieve good in vitro anti-proliferative effect, excellent inhibitory effect, and simple synthesis effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0057] The specific embodiment operates according to the following preparation method.
[0058] (1) When R 1 = R 2 When =H, prepare according to the following route:
[0059]
[0060] 1) Preparation of compound 4: dissolve compounds 1 and 2 in a small amount of dichloromethane, reflux at 100°C for 8-24 hours, cool to room temperature, and then add the reaction solution to hydrazine hydrochloride dissolved in 50% EtOH In the solution, reflux overnight at 80°C, cool to room temperature after the reaction, and wash with saturated NaHCO 3 The solution was basified, extracted with ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, spin-dried, and recrystallized with petroleum ether and ethyl acetate to obtain a white to light yellow solid, namely compound 4.
[0061] 2) Dissolving compound 4 and compound 5 in anhydrous acetic acid, heating to reflux, and completing the reaction after 8-24 hours. Cool to room temperature, spin dry the s...
Embodiment 1
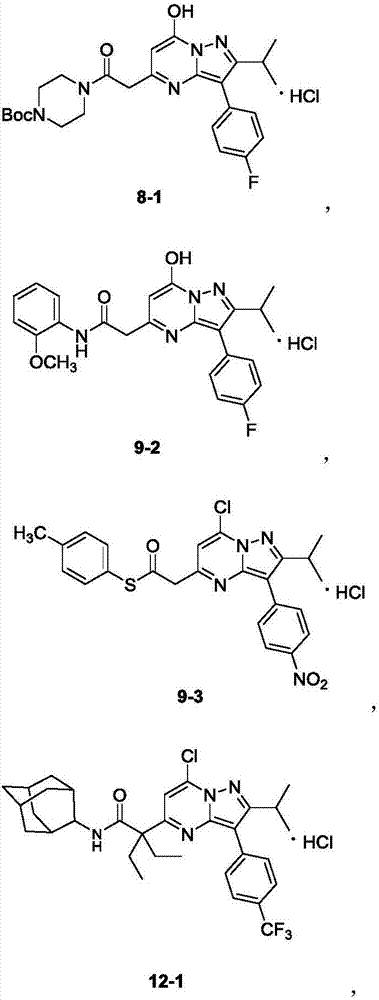
[0075] Preparation of Compound 8-1: R 1 = R 2 =H, R 4 = F, R 5 =OH,X 1 =X 2 =CH.
[0076] (1) Preparation of Compound 4: Dissolve Compound 1 (43.2mL), 2 (43.8mL) in dichloromethane (20mL) and then reflux at 100°C for 24 hours. After cooling to room temperature, add the reaction solution to into a solution of hydrazine hydrochloride dissolved in 50% EtOH (100mL), refluxed overnight at 80°C, cooled to room temperature after the reaction, and washed with saturated NaHCO 3 Basified, extracted with ethyl acetate (3*150mL), washed with saturated brine, dried over anhydrous sodium sulfate, filtered and spin-dried, recrystallized with petroleum ether and ethyl acetate to obtain a white solid, compound 4 (44.6g, 82%)
[0077] (2) Compound 4 (45.8 g) and compound 5 (36.4 mL) were dissolved in anhydrous acetic acid (300 mL), heated to reflux, and the reaction was completed after 24 hours. Cool to room temperature and spin dry the solvent. Add deionized water (300mL), and adjust...
Embodiment 2
[0081] Preparation of Compound 12-1: R 1 = R 2 =-Et, R 4 = CF 3 , R 5 =Cl,X 1 =X 2 =CH.
[0082] (1) Preparation of Compound 4: Dissolve Compound 1 (43.2mL), 2 (43.8mL) in dichloromethane (20mL) and then reflux at 100°C for 24 hours. After cooling to room temperature, add the reaction solution to into a solution of hydrazine hydrochloride dissolved in 50% EtOH (100mL), refluxed overnight at 80°C, cooled to room temperature after the reaction, and washed with saturated NaHCO 3 Basified, extracted with ethyl acetate (3*150mL), washed with saturated brine, dried over anhydrous sodium sulfate, filtered and spin-dried, recrystallized with petroleum ether and ethyl acetate to obtain a white solid, compound 4 (44.6g, 82%)
[0083](2) Compound 4 (21.8 g) and compound 5 (18.2 mL) were dissolved in anhydrous acetic acid (150 mL), heated to reflux, and the reaction was completed after 20 hours. Cool to room temperature and spin dry the solvent. Add deionized water (150mL), adj...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com