Preparation method of glutamine dipeptide for injection
A technology of alanyl glutamine and glutamine, which is applied in the field of preparation of alanyl glutamine for injection, can solve the problems of dry agglomeration, poor stability and resolubility, and low purity, and achieve impurity control Good effect, low content of related substances, uniform temperature transfer effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
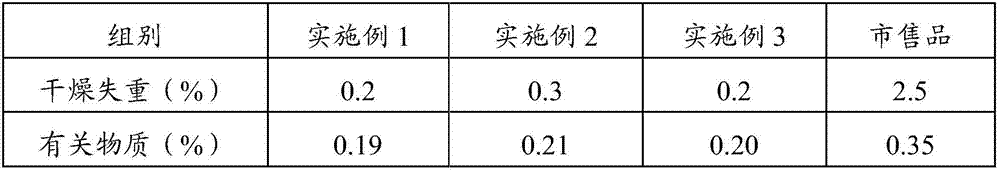
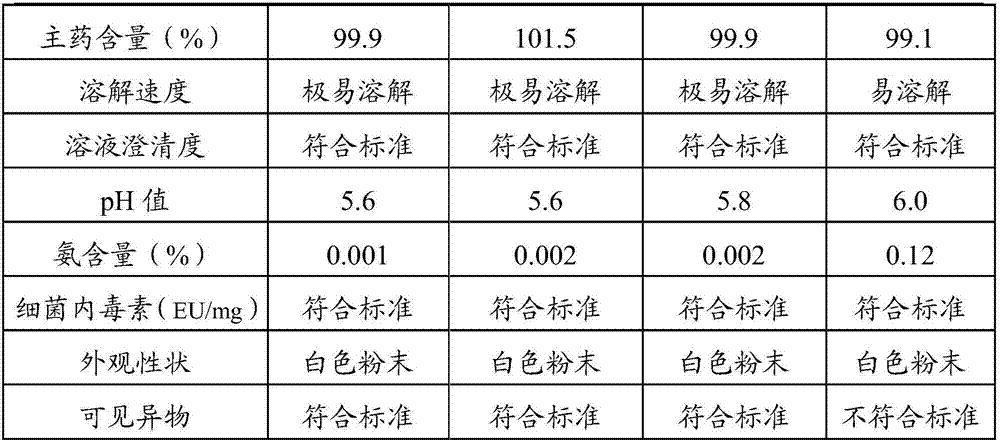
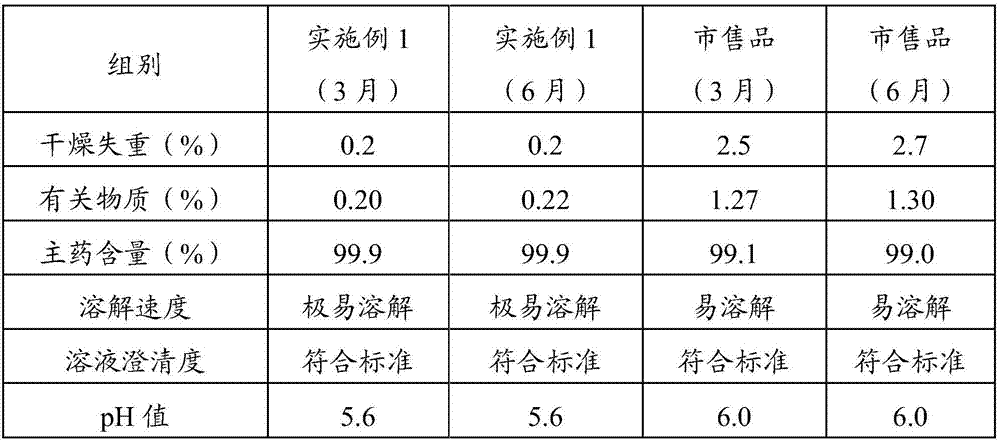
[0025] The prescription of alanyl glutamine for injection of the present invention: every 40.0kg of alanyl glutamine for injection contains 10.0kg of N(2)-L-alanyl-L-glutamine raw material, 0.4kg of activated carbon for needles And water for injection 29.6kg.
[0026] (1) Put the prescribed amount of water for injection into the liquid preparation tank, control the temperature of the water for injection to 30°C, and add the prescribed amount of N(2)-L-alanyl-L-glutamine raw material into the liquid preparation tank , stir to dissolve completely;
[0027] (2) Take the activated carbon of the prescribed amount, add a small amount of water for injection, make the activated carbon slowly absorb water to form a carbon slurry, add the carbon slurry to the medicinal solution and stir and adsorb for 10 minutes;
[0028] (3) The liquid medicine is filtered through titanium rod for carbon removal, coarse filtration with 0.45um polyethersulfone filter element, redundant filtration with ...
Embodiment 2
[0032] The prescription of alanyl glutamine for injection of the present invention: same as embodiment 1.
[0033] (1) Put the prescribed amount of water for injection into the liquid preparation tank, control the temperature of the water for injection to 20°C, and add the prescribed amount of N(2)-L-alanyl-L-glutamine raw material into the liquid preparation tank , stir to dissolve completely;
[0034] (2) Take by weighing the activated carbon of prescription quantity, add a small amount of water for injection, make activated carbon slowly absorb water and form carbon slurry, add carbon slurry in the medicinal liquid and stir and adsorb for 8 minutes;
[0035] (3) The liquid medicine is filtered through titanium rod for carbon removal, coarse filtration with 0.45um polyethersulfone filter element, redundant filtration with 0.22um polyethersulfone filter element, and sterile filtration with 0.22um polyethersulfone filter element;
[0036] (4) After the partition of the lyophi...
Embodiment 3
[0039] The prescription of alanyl glutamine for injection of the present invention: same as embodiment 1.
[0040] (1) Put the prescribed amount of water for injection into the liquid preparation tank, control the temperature of the water for injection to 40°C, and add the prescribed amount of N(2)-L-alanyl-L-glutamine raw material into the liquid preparation tank , stir to dissolve completely;
[0041] (2) Take the activated carbon of the prescribed amount, add a small amount of water for injection, make the activated carbon slowly absorb water to form a carbon slurry, add the carbon slurry to the medicinal solution and stir and adsorb for 12 minutes;
[0042] (3) The liquid medicine is filtered through titanium rod for carbon removal, coarse filtration with 0.45um polyethersulfone filter element, redundant filtration with 0.22um polyethersulfone filter element, and sterile filtration with 0.22um polyethersulfone filter element;
[0043](4) After the partition of the lyophil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com