A heat-resistant freeze-drying protective agent of swine fever live vaccine
A swine fever live vaccine and heat-resistant freeze-drying technology are applied in the field of heat-resistant freeze-drying protective agents, which can solve problems such as energy waste and cost increase, and achieve the effects of reducing side reactions, preventing denaturation and protecting activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Embodiment 1, a kind of heat-resistant lyoprotectant of swine fever live vaccine is characterized in that: it is formulated by the raw material of following parts by weight: 2% inositol, 1% pendant calendula alcohol, 10 % sucrose, 1% gum arabic, 0.15% sarcosine, 0.05% vitamin C, and the balance is water for injection.
[0014] The preparation method of heat-resistant lyoprotectant of the present invention is as follows:
[0015] Step 1) Dissolve inositol, calendula alcohol, sucrose, and gum arabic in 800ml of water for injection in proportion, and sterilize at 100°C for 30 minutes to obtain solution 1, which is set aside;
[0016] Step 2) Dissolve sarcosine and vitamin C in 100ml of water for injection, filter through a 0.22um filter membrane to obtain solution 2, and set aside;
[0017] Step 3) Combine solution 1 and solution 2, and add water for injection to 1000ml, which is the heat-resistant lyoprotectant of the present invention.
Embodiment 2
[0018] Embodiment 2, the application of heat-resistant lyoprotectant
[0019] (1) Mix the above-mentioned heat-resistant lyoprotectant with the suspension of classical swine fever virus at a ratio of 1:1, dispense quantitatively, and freeze-dry to make a live vaccine for swine fever.
[0020] (2) At the same time, a commercially available swine fever live vaccine (rabbit source) of a certain brand was selected as a control vaccine.
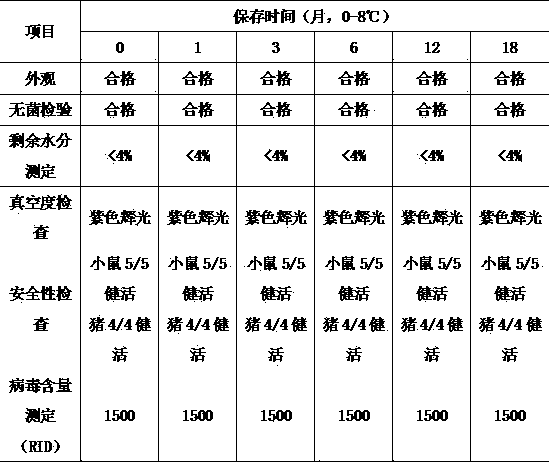
[0021] (3) Vaccine inspection
[0022] The above-mentioned commercially available swine fever live vaccine of a certain brand and the protective agent vaccine of the present invention were stored in a cold storage at 2-8°C, respectively, in 0 months, January, March, June, December, and 18 months, according to the 2015 version of veterinary medicine Code-related items are inspected, and the inspection results are inspected. In order to be closer to actual production, the two vaccines were placed in a 22°C incubator for 7 days to carry out relevan...
Embodiment 3
[0030] Embodiment 3, a kind of heat-resistant lyoprotectant of swine fever live vaccine is characterized in that: it is formulated by the raw material of following parts by weight: 1% inositol, 0.5% pendant calendula alcohol, 5% % sucrose, 0.5% gum arabic, 0.05% sarcosine, 0.01% vitamin C, and the balance is water for injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com