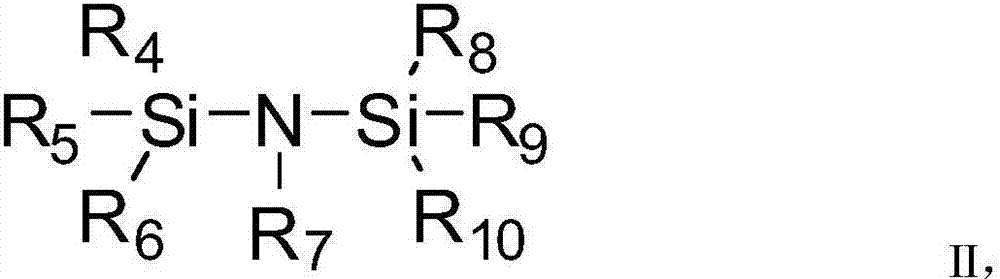Non-aqueous electrolyte and high-nickel ternary cathode material battery
An electrolyte and lithium-ion battery technology, applied in electrical components, secondary batteries, circuits, etc., can solve the problems of limited improvement of cycle performance of high-nickel ternary cathode materials, capacity decay, and deterioration of material thermal stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~10
[0092] The organic solvent is selected from carbonate solvents, that is, a mixture of ethylene carbonate EC and dimethyl carbonate DMC, and its volume ratio is 3:7.
[0093] Lithium salts were selected from LiPF 6 and the N-atom-centered lithium salt LiTFSI, so that the molar concentration is 1mol L -1 .
[0094] Additive A is selected from the above formulas 1-9, additive B is preferably LiPF 6 (mass percentage is 2%), the additive C is preferably 1,3-propane sultone (PS, mass percentage is 1%), and finally the lithium-ion battery electrolyte is obtained.
Embodiment 11
[0099] Parallel performance detection is carried out to the electrolyte solutions shown in Examples 1-10 of the present invention and Comparative Examples 1-2,
[0100] see figure 1 , figure 1 It is the appearance diagram of the 1000mAh soft-pack laminated battery used for testing in the embodiment of the present invention and the comparative example.
[0101] The lithium-ion battery electrolytes prepared above were respectively injected into 1000mAh soft-pack laminated batteries. carry out testing.
[0102] The positive electrode is high-nickel ternary material NCM nickel cobalt lithium manganese oxide (811), the negative electrode is artificial graphite, and the diaphragm is Celgard2075.
[0103] Test conditions: charge and discharge voltage range is 2.5 ~ 4.3V;
[0104] Normal temperature cycle performance: 200 charge and discharge cycles at 30°C and 1C rate at room temperature;
[0105] High temperature performance test: charge and discharge cycle 100 times at 60°C hi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com