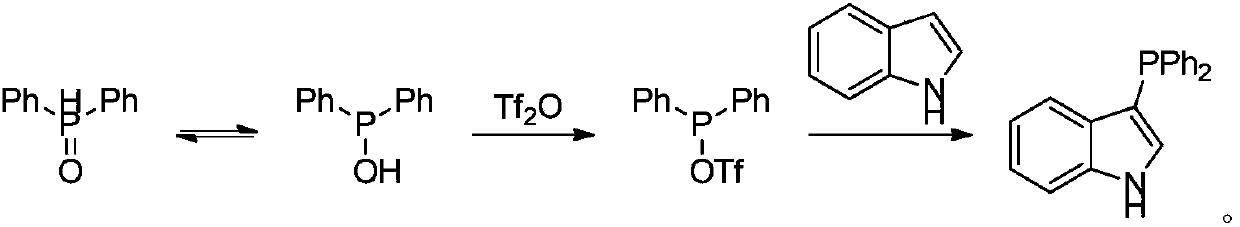
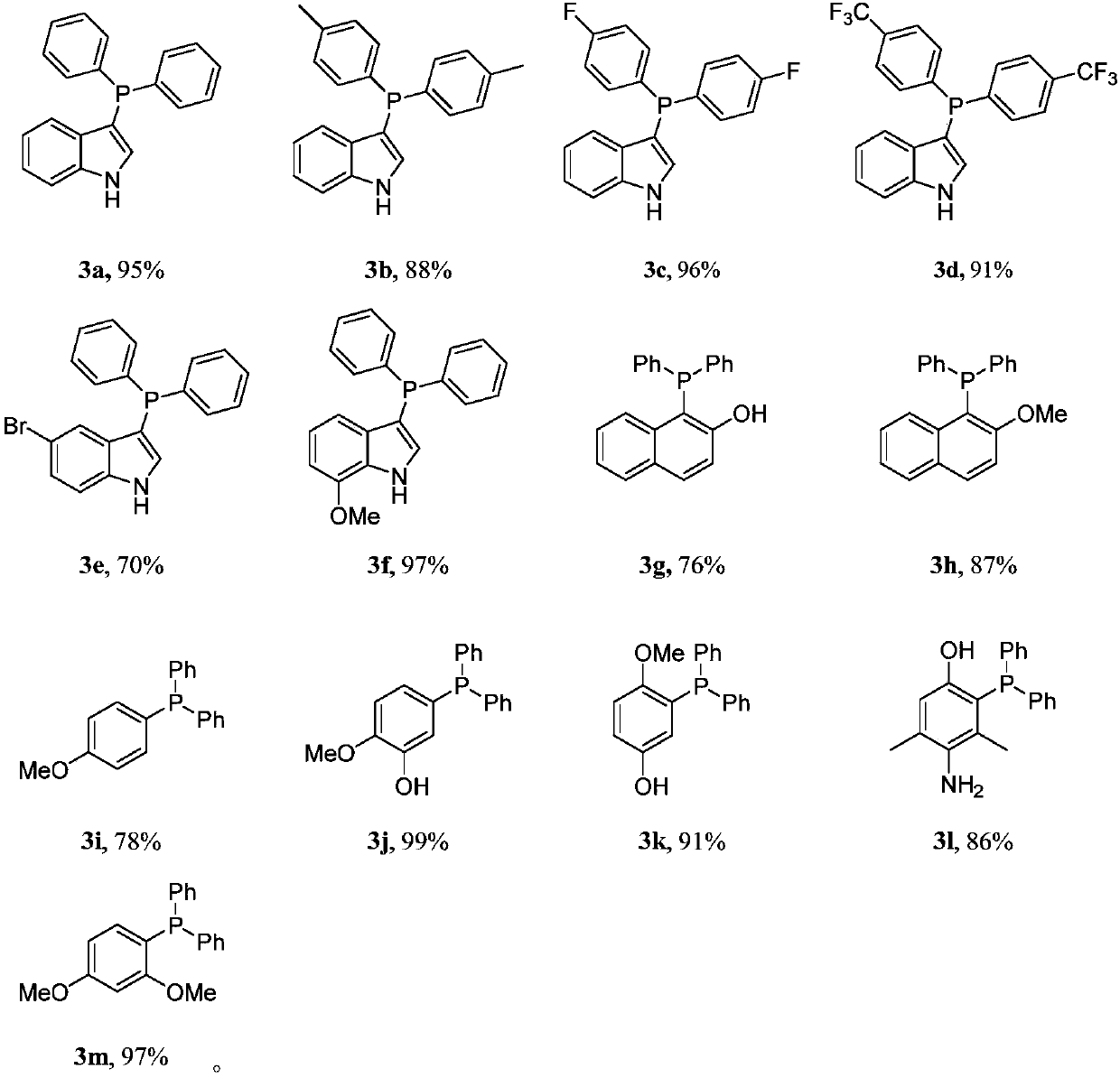
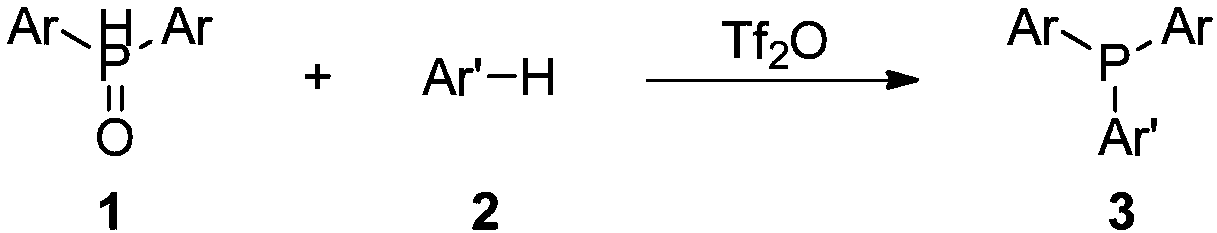The synthetic method of triaryl phosphine
A technology of triarylphosphine and synthesis method, which is applied in the field of synthesis of triarylphosphine, can solve problems such as increased difficulty in reaction operation, harsh reaction conditions, and expensive raw materials, and achieves scale-up production, good substrate tolerance, and excellent reaction conditions simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0018] Example: Preparation of Triarylphosphine 3
[0019]
[0020] Add 0.75mmol of diarylphosphine oxide 1, 0.50mmol of electron-rich arene 2, 2.0mL of acetonitrile and 0.75mmol of trifluoromethanesulfonic anhydride into a 10mL single-necked flask, and react at 80°C for 12h. After the reaction, cool to room temperature, remove the solvent by rotary evaporation, and finally obtain the target product triarylphosphine 3a-3m through column chromatography on silica gel, and the yield is as follows.
[0021]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com