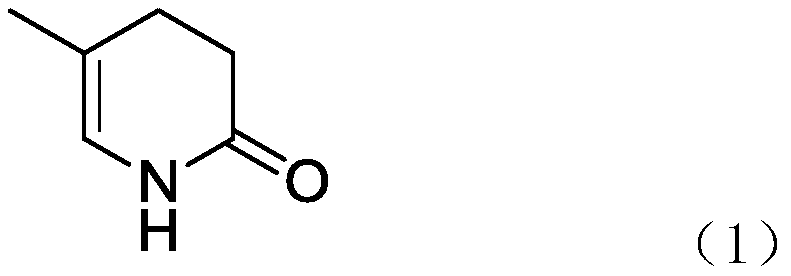
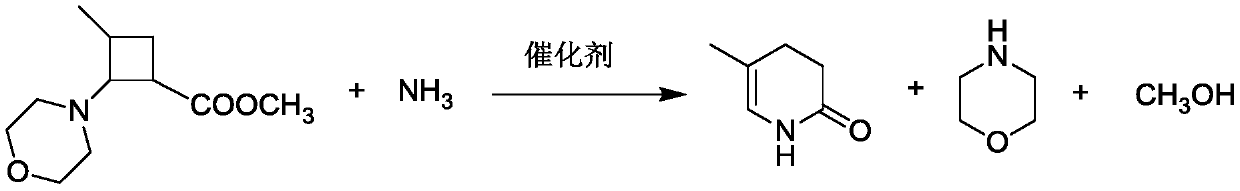
A method for catalytically synthesizing 5-methyl-3,4-dihydropyridin-2(1h)-one chemical intermediates
A technology of dihydropyridine and methyl is applied in the field where methyl 3-methyl-2-cyclobutyrate is used as a raw material, and can solve the problems of difficulty in meeting environmental protection requirements, difficulty in recovering morpholine, etc., and achieves low production cost, Good catalytic effect, easy to recycle and apply
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 340.41g of cyclobutane (content 80.37%), 872.85g of 15% ammonia water (the molar ratio of ammonia water to cyclobutane is 6:1) and 10.21g of iron powder (the amount of catalyst is 3% of the amount of cyclobutyl) Into a 2L autoclave, control the stirring speed to 400r / min, and keep the reaction at 60°C for 3h. Subsequently, the obtained reaction kettle liquid was subjected to suction filtration, and the filter cake was washed several times with a small amount of 155.47 g of water to obtain 25.38 g of wet catalyst and 1365.15 g of filtrate. Then the filtrate was distilled under normal pressure to recover 1031.85 g of ammonia water with a concentration of 9.78%, and 192.43 g of morpholine was recovered through negative pressure distillation with a concentration of 49.16%. Finally, the still material was distilled under high vacuum to obtain 134.51 g of crude pyridone, with a content of 74.24%. The crude pyridone was recrystallized from chlorobenzene and dried to obtai...
Embodiment 2-5
[0027] On the basis of Example 1, ammoniacal liquors of different concentrations were adopted (the number of moles was constant), and other conditions were constant. The obtained results are shown in Table 1.
[0028] The impact of the different ammonia concentrations of table 1 on the product yield
[0029] Example number Ammonia concentration / % Pyridone Yield / % Example 2 5 60.14 Example 3 10 63.87 Example 4 20 64.51 Example 5 25 62.95
Embodiment 6-10
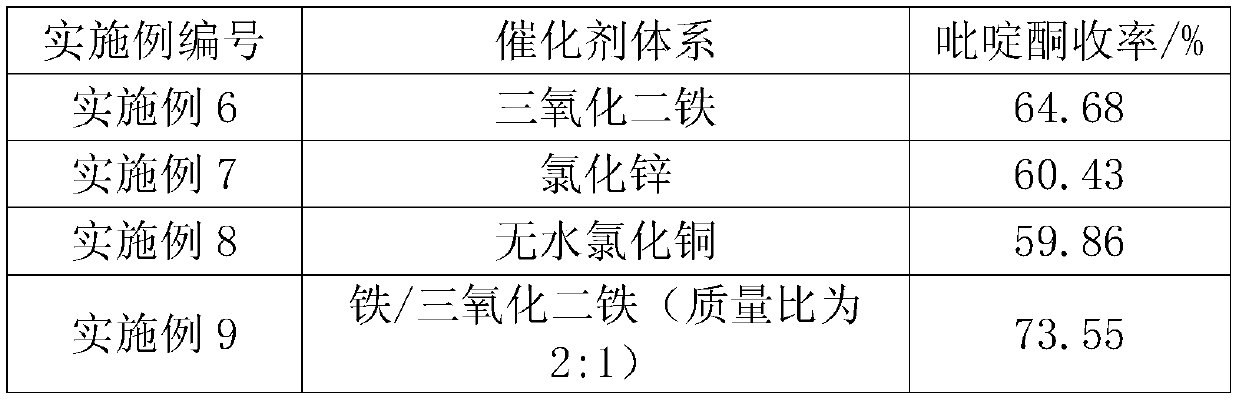
[0031] On the basis of Example 1, different catalyst systems were used, and other conditions remained unchanged. The obtained results are shown in Table 2.
[0032] The impact of different catalyst systems on the product yield in table 2
[0033]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com