Building method of acute hyperuricemia mouse model
A technology of hyperuricemia and mouse model, which is applied in pharmaceutical formulations, organic active ingredients, medical preparations containing active ingredients, etc., can solve the problems of low molding rate, increased mortality, etc. The effect of strong reproduction and easy feeding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
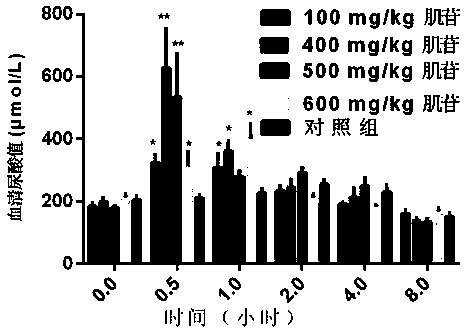
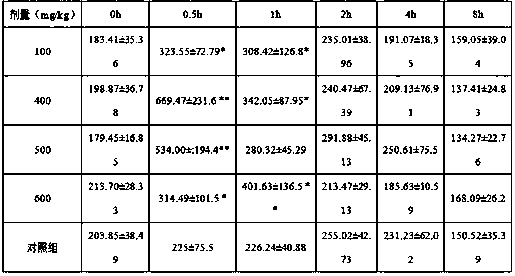
[0046] Example 1: Experiment on effectiveness and dose-effect relationship of inosine-induced acute hyperuricemia in mice
[0047] Select 40 mice of 22-25g, half male and half male, and randomly divide them into 5 groups, 8 mice in each group, and inject inosine intraperitoneally according to the following doses: 100mg / kg, 400mg / kg, 500mg / kg, 600mg / kg kg, and the control group was an equal volume of normal saline. Take 0.5ml of blood from the tail vein at 0h, 0.5h, 1h, 2h, 4h, and 8h after medication, separate the serum, and measure the serum uric acid value with the phosphotungstic acid method. .
[0048] The results showed that compared with the control group, the uric acid value of the 100mg / kg, 400mg / kg, 500mg / kg, and 600mg / kg dosage groups of mice increased at 0.5h. Compared with the control group, the uric acid value in the 100mg / kg dose group of mice increased, but the increase was not large. The blood uric acid concentration of the 400mg / kg and 500mg / kg dose groups w...
Embodiment 2
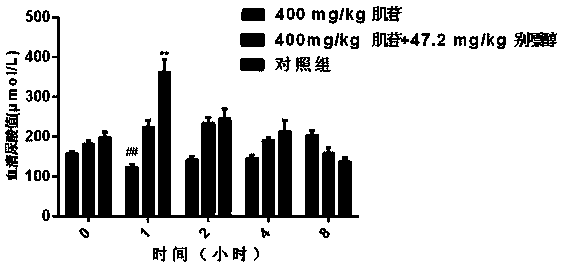
[0052] Example 2: Application of Acute Hyperuricemia Mouse Model in the Screening of Drugs for Lowering Uric Acid - Effect of Allopurinol on Blood Uric Acid in Hyperuricemia Mice
[0053] Allopurinol is an inhibitor of xanthine oxidase and is currently a commonly used urate-lowering drug. The optimal dose of allopurinol for reducing the uric acid level in mice with hyperuricemia caused by oxonate potassium is 47.2 mg / kg, which is also reported in the literature. In order to further verify that this dose can also effectively reduce hyperuricemia caused by inosine For the uric acid value of mice, 24 normal ICR mice, 22-25g, half male and half male, were randomly divided into 3 groups, 8 animals in each group, injected intraperitoneally, respectively 400mg / kg inosine group, 400mg / kg muscle Glycoside+47.2mg / kg allopurinol group, equal volume of normal saline control group. At 0h, 1h, 2h, 4h, and 8h after administration, 0.5ml of blood was collected from the tail vein, the serum w...
Embodiment 3
[0058] Example 3: Preliminary Toxicity and Safety Research of Inosine on Mice:
[0059] In this embodiment, different groups of mice after the mouse effectiveness experiment and the dose-effect dependence experiment of the above-mentioned embodiment 1 were respectively tested (see Example 1 for experimental grouping: 40 mice of 22-25 g were selected, half male and half male, randomly divided into two groups) 5 groups, 8 rats in each group, intraperitoneal injection of inosine according to the following doses, the doses are: 100mg / kg, 400mg / kg, 500mg / kg, 600mg / kg, the control group is equal volume of normal saline), continuous observation for 15 On the first day, the animal poisoning and death conditions were recorded. The results showed that all the animals did not show any discomfort, moved freely, took water and food, and the urine and stool were normal, and the general condition was good. All animals were sacrificed 15 days after the intraperitoneal injection, and grossly d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com