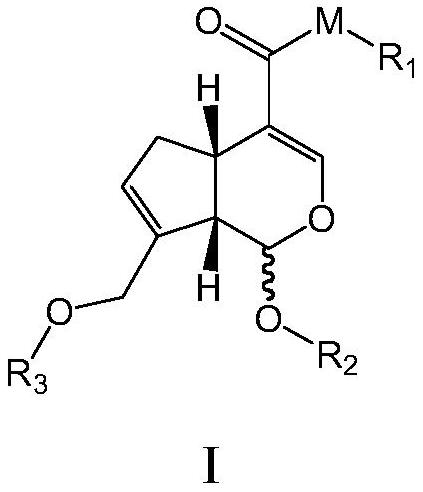
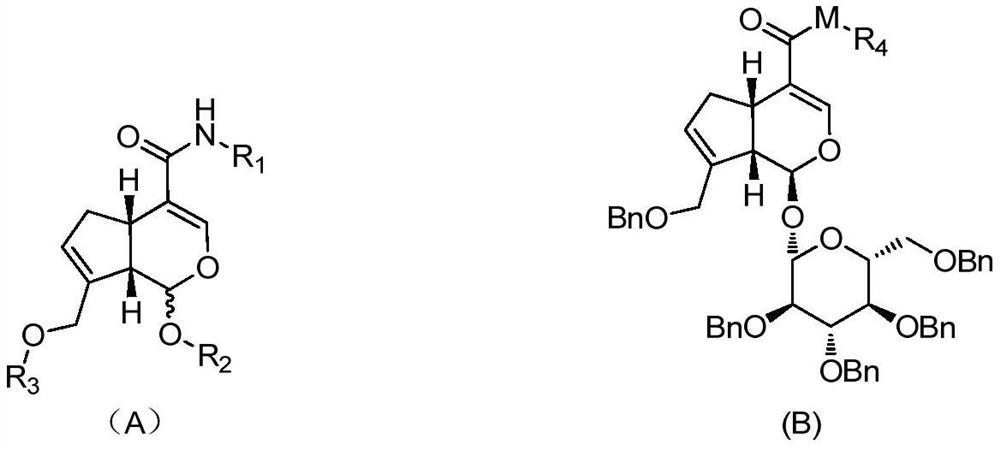
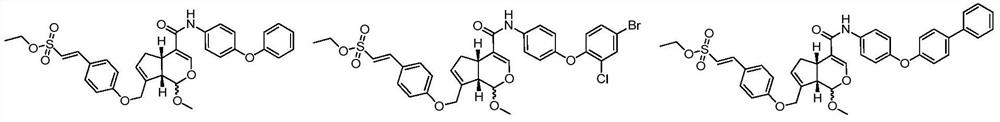
A class of genipin derivatives and their preparation and application
A technology of genipin and derivatives, which is applied in the field of novel genipin derivatives, and can solve the problem that the inhibitory activity of PTP1B protein is not obvious
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0084] Preparation of genipin derivatives
[0085] The present invention also provides a method for preparing the above-mentioned compound of formula I, said method comprising the steps of:
[0086] In a preferred embodiment, the method for the genipin derivative shown in the formula (A) may further comprise the steps:
[0087] (1) Take genipin and add it to a certain volume of R 2 -OH, stirring at a certain temperature, and adding a few drops of concentrated hydrochloric acid to catalyze the compound shown in formula (II)
[0088] In another preferred example, the temperature is preferably 60-100°C.
[0089] (2) get 1 molar equivalent of the compound shown in formula (II) 2 molar equivalents of triethylamine and dichloromethane are added to a round bottom flask, under an ice-water bath, 1.5 molar equivalents of chlorination reagent are added, a certain amount of 4-dimethylaminopyridine (DMAP) is catalyzed, and the reaction is carried out at room temperature to obtain C...
Embodiment 1
[0125] Synthesis of 1-methoxygenipin
[0126] The specific steps of this example include: dissolving genipin (10 g, 44 mmol) in methanol (70 ml), adding two drops of concentrated hydrochloric acid to the solution for catalysis, and heating to reflux for 7 hours. After the reaction, concentrate under reduced pressure, add a few drops of 1N sodium hydroxide solution to adjust the pH to neutral, extract three times with ethyl acetate, combine the organic phases, wash the organic phases with saturated brine three times, and dry the organic phases with anhydrous sodium sulfate . Concentration under reduced pressure gave 1-methoxygenipin (10.620 g, 44 mmol) as a brown oily liquid with a yield of 98%. 1 H NMR (400MHz, CDCl 3 ): δ7.41-7.54(m,1H),5.75-5.88(m,1H),4.46(d,J=8.2Hz,1H),4.12-4.28(m,2H),3.64-3.75(m,3H ),3.40-3.54(m,3H),3.13-3.23(m,1H),2.86(dd,J=8.5,16.5Hz,1H),2.59(t,J=7.6Hz,1H),2.06(dd, J=9.0,16.4Hz,1H).
Embodiment 2
[0128] Synthesis of 1-methoxy-2-(chloromethyl)genipin
[0129] The specific steps of this example include: dissolving 1-methoxygenipin (10.620g, 44mmol) and triethylamine (8.905g, 88mmol) in dichloromethane (70ml), slowly adding benzenesulfonyl chloride dropwise in an ice-water bath (11.656g, 66mmol), after the dropwise addition was completed, DMAP (200mg) was added to catalyze the reaction at room temperature for 40 hours. After the reaction was completed, 1N sodium hydroxide solution (10 ml) was added for extraction three times, and the organic phases were combined. 1N hydrochloric acid solution was added to the organic phase to adjust the pH to neutral, the extracted organic phase was washed three times with saturated brine, and the organic phase was dried over anhydrous sodium sulfate. After concentration under reduced pressure, purification by silica gel column chromatography (petroleum ether: ethyl acetate = 15:1) gave 1-methoxy-2-(chloromethyl)genipin (5.603g, 22mmol) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com