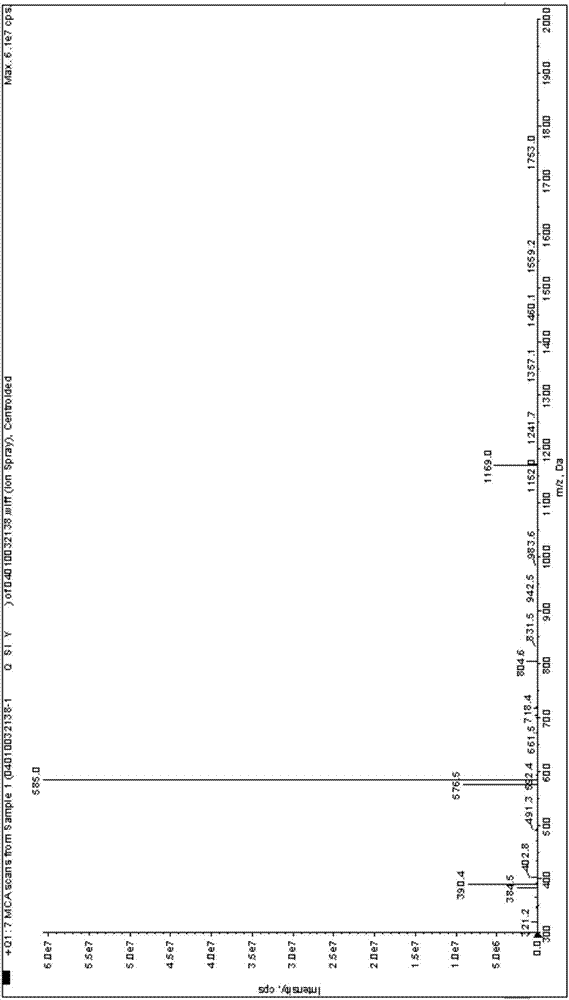
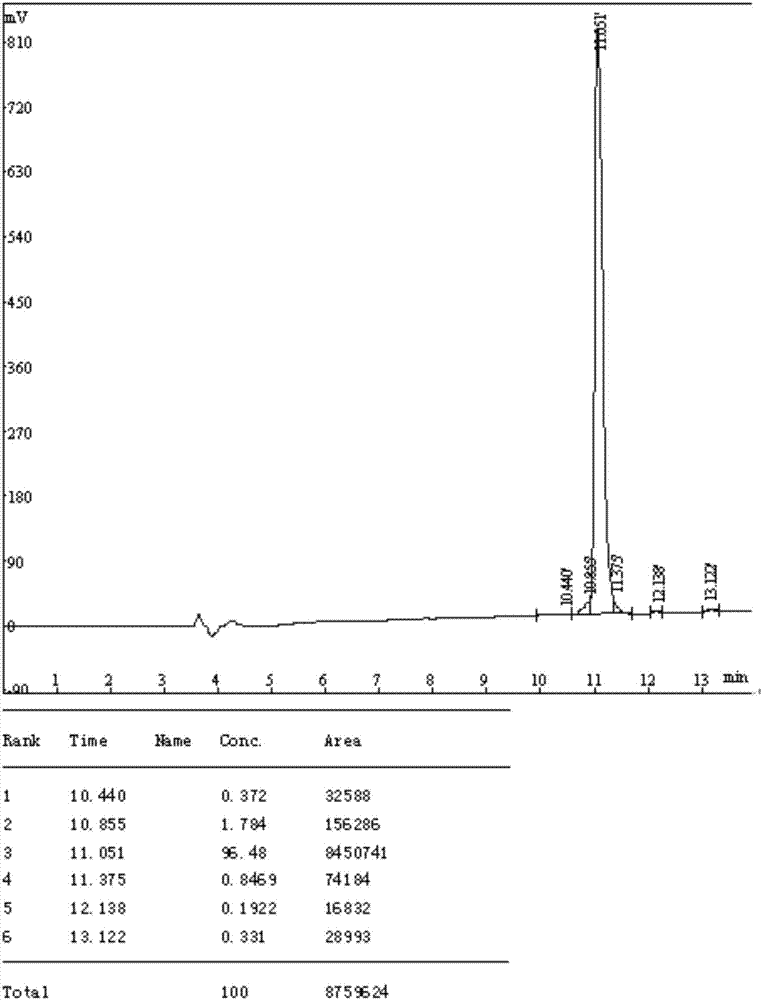
Preparation method of farnesyl-modified cysteine polypeptide
A cysteine and amino acid technology, applied in the field of cysteine polypeptide synthesis, can solve problems such as difficulty in determining the farnesyl reaction site, affecting yield and structure determination, difficulties in later purification and identification, etc., to achieve Less impurities, definite structure, avoid side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The synthesis of H-Met-Glu-Asp-Pro-Ala-Cys(farnesyl)-Val-Ile-Ser-COOH comprises the following steps: synthesis from the C-terminus of the polypeptide to the N-terminus:
[0062] 1. Weigh 0.4mmol / g Wang Resin 2g (synthesis scale is 0.8mmol) and put it into the reaction tube, add DMF (15ml / g), and blow nitrogen for 30min to make it fully swell.
[0063] 2. The first amino acid connected to the polypeptide: filter the solvent through a sand core, add 0.46g Fmoc-Ser(tBu)-OH and dissolve it completely with an appropriate amount of DMF, add 2.4mmol DIC, then add 0.08mmol DMAP, react with nitrogen for 120min, pyridine : Acetic anhydride = 1:1 capping for 30 minutes. Fmoc-Ser(tBu)-Resin was obtained.
[0064] 3. Remove the Fmoc protecting group: remove the reaction solution, add 20% piperidine / DMF solution (15ml / g) for 5min, remove the reaction solution, and add 20% piperidine / DMF solution (15ml / g) for 15min. The piperidine solution was sucked off, and the resin was washed 6 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com