Preparation method of alarelin
A kind of technology of alanorelin and alanorelin is applied in the preparation method of peptide, chemical instrument and method, organic chemistry and other directions, and can solve the problems such as the preparation method of alanorelin that has not been disclosed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
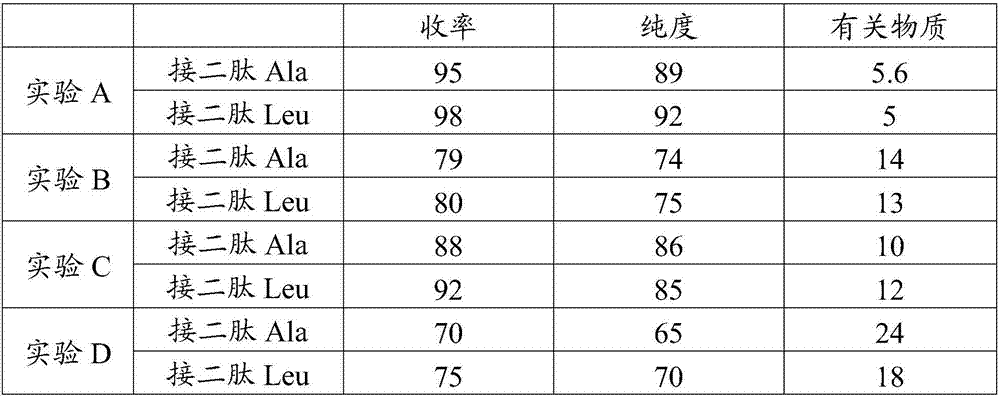
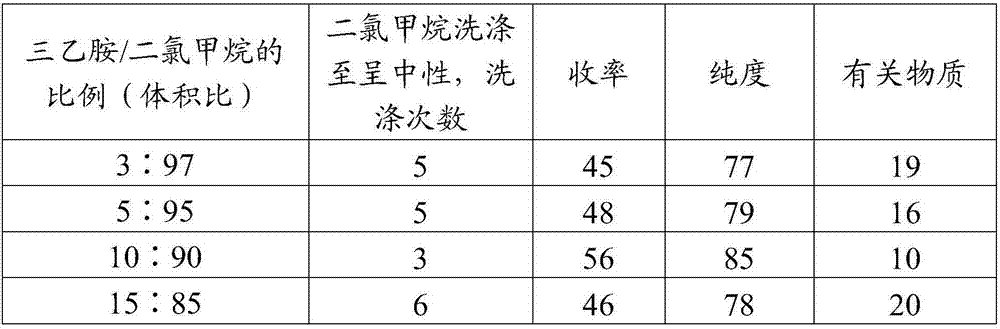
Image
Examples
Embodiment 1
[0027] Activate Boc-Pro
[0028] Weigh 193.8g of Boc-Pro in a three-necked flask and dissolve it in 1200ml of methanol.
[0029] Weigh 190.6g of Cs in the three-necked flask 2 CO 3 Soluble in 1200mlH 2 O was slowly added to the above Boc-Pro solution to obtain a colorless and transparent Boc-Pro-Cs solution with a pH value of 7.2.
[0030] Concentrate under reduced pressure with a water pump in a water bath at 40°C, evaporate the solvent to obtain a solid; add 500ml of methanol, evaporate to dryness under reduced pressure, add 500ml of methanol again and evaporate to dryness under reduced pressure to obtain 400g of dry Boc-Pro-Cs.
Embodiment 2
[0032] Boc-Pro curing
[0033] Boc-Pro-Cs was prepared according to the method described in Example 1.
[0034] Weigh 670g of chloromethyl resin, 400g of Boc-Pro-Cs and 2000ml of DMF, dissolve them completely and pour them into a three-neck flask.
[0035] Stir and react in a water bath at 45°C for 48 hours, filter with suction, wash the resin 3 times with DMF, then wash with purified water until no Cl- reaction occurs, wash 4 times with absolute ethanol, and wash 2 times with methanol. Dry in an oven at 40-45°C to obtain dried Boc-Pro resin.
[0036] The substitution equivalent number of Boc-Pro is 0.92mmol / g, and the reaction yield is 96%.
Embodiment 3
[0038] Boc-Pro curing
[0039] Boc-Pro-Cs was prepared according to the method described in Example 1.
[0040] Weigh 670g of chloromethyl resin, 300g of Boc-Pro-Cs and 1800ml of DMF, and pour them into a three-necked flask after completely dissolving.
[0041] Stir and react in a water bath at 45°C for 48 hours, filter with suction, wash the resin 3 times with DMF, then wash with purified water until no Cl- reaction occurs, wash 4 times with absolute ethanol, and wash 2 times with methanol. Dry in an oven at 40-45°C to obtain dried Boc-Pro resin.
[0042] The substitution equivalent number of Boc-Pro was 1.0 mmol / g, and the reaction yield was 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com