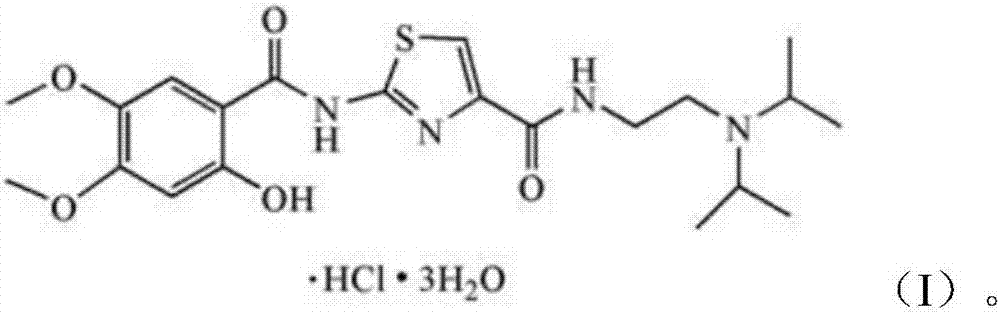
Medicine tablets for treating dyspepsia and preparing method of medicine tablets
A technology for indigestion and tablets, which is applied to the digestive system, drug combination, pill delivery, etc., can solve the problem of poor fluidity of acotiamide hydrochloride capsules, poor dissolution of acotiamide hydrochloride capsules, and increased water content. and other problems to achieve the effect of improving safety and effectiveness, good fluidity, and reduced hygroscopicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
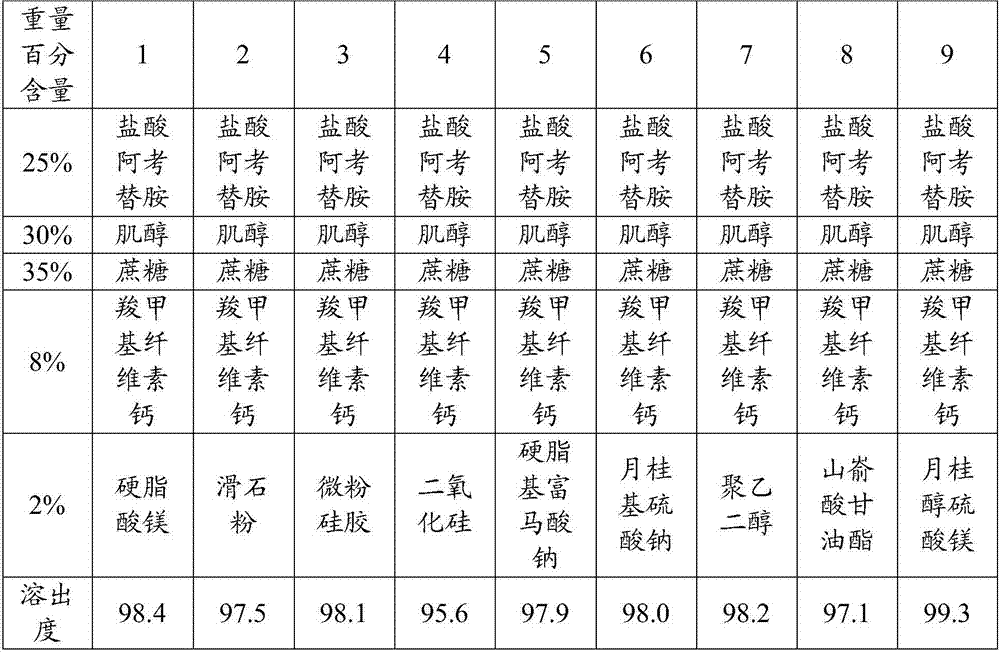
[0056] Experimental Example 1: Lubricant Type Screening Test
[0057] In this test, the lubricant described in the present invention is investigated to investigate the influence of the lubricant type on the dissolution rate of the product. Tablets were prepared by the preparation method of the present invention, and only part of the experimental data is listed here; the screening results are shown in Table 1.
[0058] Table 1 Results of screening experiments for lubricant types
[0059]
[0060] As can be seen from this, lubricants have a great influence on the dissolution rate of acotiamide hydrochloride tablets, commonly used lubricants such as magnesium stearate, talcum powder, micropowder silica gel, silicon dioxide, sodium stearyl fumarate , sodium lauryl sulfate, polyethylene glycol, glyceryl behenate, etc., the dissolution rate of acotiamide hydrochloride tablets obtained from them is relatively low.
[0061]
[0062]
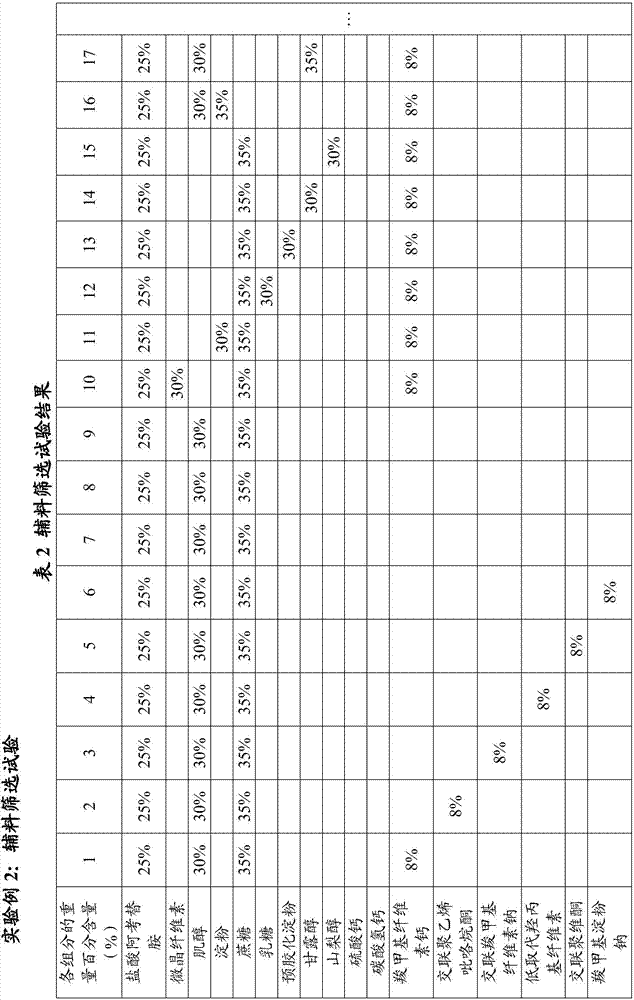
experiment example 3
[0063] Experimental Example 3: Performance Testing
[0064] The comparison of the performance of acotiamide hydrochloride tablets prepared according to the prescription provided by the invention and the preparation method and the performance of prior art acotiamide hydrochloride tablets is shown in Table 3:
experiment example 4
[0085] Experimental Example 4: Accelerated Test
[0086] Get the samples of Examples 1 and 2 of the present invention and place them for 6 months at a temperature of 40°C ± 2°C and a relative humidity of 75% ± 5%. Take a sample at the end of the 1st, 2nd, 3rd, and 6th months respectively. Sex key investigation items are measured. The test results are shown in Table 4.
[0087] Table 4 Accelerated test results
[0088]
[0089]
[0090] Carry out identical experiment to other embodiment of the present invention, obtain the result similar to embodiment 1, 2 of the present invention; As can be seen from the experimental result, the product of embodiment of the present invention is 75% ± 75% ± 2 ℃ at temperature 40 ℃ ± 2 ℃, relative humidity. Under the condition of 5%, it has high stability in terms of dissolution rate, related substances, and crystal form.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Outer diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com