Bionic bleeding stopping biological glue
A hemostatic material and protein technology, applied in the field of bionic hemostatic biological glue, can solve problems such as difficult dressing changes, wound adhesion, and inadaptability to emergency bleeding emergencies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
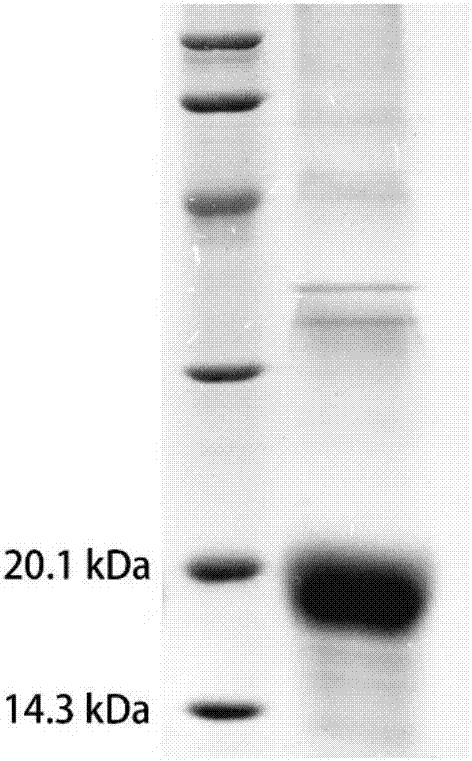
[0023] Example 1 protein EGFL sbp9 Methods for in vitro expression of repetitive sequences.
[0024] By analyzing the scallop foot transcriptome, the present invention discovered the protein Sbp9 with cell adhesion function, and found a protein with EGF repeat sequence, named EGFL sbp9 , its amino acid sequence is as follows:
[0025] APCGGSCKANEHCDMHSQECVCNTGYKLYKKACVLPCGGPCKQYERCDEGSNKCVCMTGYSLFKGSCVVPCGGPCGPNAYCDKNKNQCNCNKGYFTYHGVCALPCGGPCKQNANCDKNSNQCVCNKGYK EIGGVCAV;
[0026] 1. EGFL sbp9 expression
[0027] The gene fragment was connected to pET28a by restriction endonuclease, and transformed into Escherichia coli BL21(DE3) to obtain a recombinant expression system. Grow it to OD using LB medium 600 When the concentration is 0.6-0.8, add IPTG to make the final concentration 0.2mM, and induce overnight. The cells were collected by centrifugation and stored at -80°C.
[0028] 2. EGFL sbp9 separation and purification of
[0029] The cells were resuspended in PBS an...
Embodiment 2
[0030] The chemical synthesis of embodiment 2 sodium alginate graft Dopa (Dopa-SA)
[0031] A Dopa-modified sodium alginate derivative was synthesized and named as Dopa-SA.
[0032] Weigh 0.2 g of sodium alginate and dissolve it in 15 mL of 0.1 M MES buffer solution with pH 6.3, stir at room temperature until dissolved, it takes about 1 h, and the final concentration is 1% (w / v). After the sodium alginate is completely dissolved, add 0.1162g NHS and 0.2903g EDC, keep the molar ratio of EDC to sodium alginate at 1.5-2.5:1, and keep the molar ratio of NHS to sodium alginate at 1.5-2.5:1 , stirred well at room temperature for 1 h.
[0033] Add 5ml of MES buffer solution with pH 6, add 0.2872g of dopamine hydrochloride, keep the molar ratio of dopamine to sodium alginate at 1.5:1, stir and mix at room temperature. After the dopamine hydrochloride was dissolved in the sodium alginate solution, the measured pH of the solution was 6.0 and stirred slowly at room temperature for 24 h...
Embodiment 3
[0034] Embodiment 3: the preparation of gel
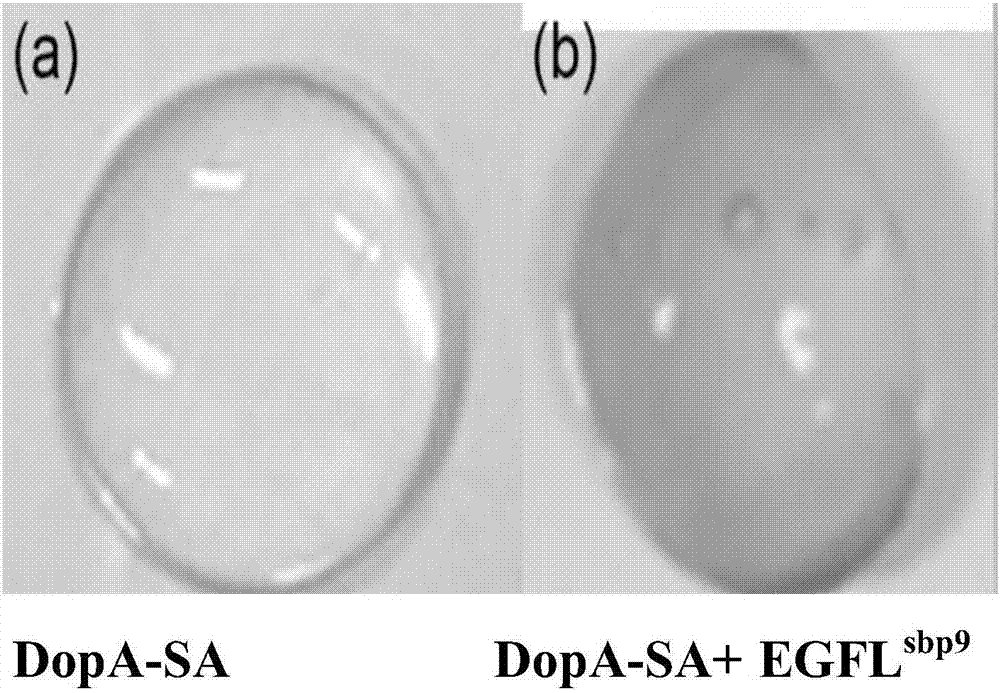
[0035] The modified Dopa-SA was prepared into a solution with a concentration of 2% (wt) in PBS solution, stirred and dissolved at 4°C for 24 hours. Then centrifuge at 6000g for 10min to remove air bubbles. Take 200μl sample and drop it on the cover glass, add the prepared EGFL in proportion (molar ratio 5-10:1) sbp9 Recombinant protein preparation gel. It was found that after adding EGFL sbp9 After 5min, a water-insoluble bioglue with certain adhesion properties was obtained ( figure 2 ).
[0036] 1. Ultrastructural observation of bioglue
[0037] Take 1ml of DopA-SA and put it on the glass slide, add EGFL according to the ratio (molar ratio 5-10:1) sbp9 , stir well. After gel formation, the hydrogel was transferred into a 24-well plate and placed at -80°C for 24 hours, and then the frozen sample was subjected to vacuum freeze-drying. The freeze-dried hydrogel is white and fluffy, and the freeze-dried sample is quickly cu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com