Antibody composition for TNF-alpha, and application thereof
An antibody composition and the technology of the composition are applied in the directions of antibodies, drug combinations, medical preparations of inactive ingredients, etc., can solve problems such as discomfort and pain, and achieve the effect of reducing pain and discomfort
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Embodiment 1: Preparation of antibody composition
[0075] This embodiment gives an example to the preparation of the preparation A of 20L:
[0076] (1) Weigh out the following ingredients: 840g mannitol (stabilizer), 20g polysorbate 80 (surfactant) and 20L water for injection.
[0077] Carbon dioxide is charged after the preparation of the formulation is completed.
[0078] (2) Dissolving the weighed mannitol (stabilizer) and polysorbate 80 (surfactant) in about 90% by weight of the water for injection to prepare a solvent system.
[0079] It has been proved that the order of adding mannitol (stabilizer) and polysorbate 80 (surfactant) does not affect the quality of the preparation and can be selected at will.
[0080] (3) Thaw the BAT1406 antibody concentrate containing 2.0 kg of total protein in a water bath, and then add the solvent system to the antibody concentrate under stirring until the final weight of the total solution is reached.
[0081] (4) Fill carbon ...
Embodiment 2
[0085] Embodiment 2: Stability study under room temperature condition
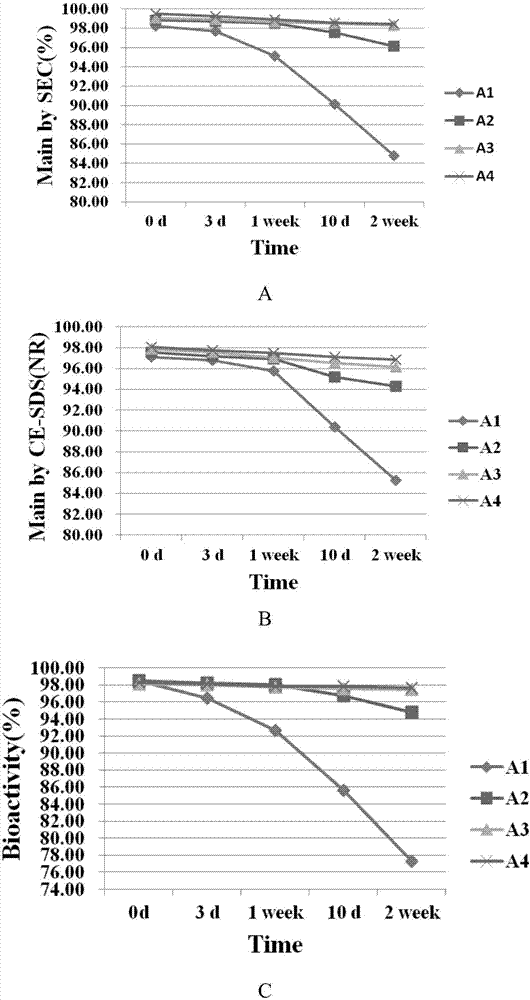
[0086] Prepare PBS sample A1 (20mg / mL) of BAT1406 antibody and low-concentration sample A2 (4mg / mL), medium-concentration sample A3 (20mg / mL), high-concentration sample A4 (100mg / mL) of preparation A, and place at room temperature Keep for 2 weeks. The formula composition of A1-A4 number sample is shown in Table 2A:
[0087] Table 2A
[0088]
[0089] The samples were tested by capillary electrophoresis (capillary electrophoresis, CE), size-exclusion chromatography (size-exclusion chromatography, SEC) and biological activity (TNF-α-induced L929 cytotoxicity neutralization experiment). The results of the determination are shown in Table 2B and figure 1 .
[0090] The results showed that: samples with three concentrations of BAT1406 antibody A preparation, low (A2 sample), medium (A3 sample), and high (A4 sample), were placed at room temperature for 2 weeks, and the degradation trend of the main peak...
Embodiment 3
[0094] Embodiment 3: Study on factors affecting pH of preparation
[0095] Prepare 10 L of samples of BAT1406 antibody A preparation (see Table 1A for the specific formula) with a concentration of 100 mg / mL, store in a pressure tank, fill with different amounts of carbon dioxide, and place it at 4 ° C. When detecting different carbon dioxide pressures, the pH of the preparation in the pressure tank . The results of the assay are listed in Table 3A.
[0096] The carbon dioxide gas pressure of table 3A.BAT1406 A preparation influences the experimental result on pH
[0097] Carbon dioxide pressure (mmHg)
Formulation pH
300
4.76
270
4.98
240
5.06
210
5.15
180
5.30
150
5.51
100
5.84
[0098] Prepare BAT1406 antibody A preparation (see Table 1A for specific formula) with a concentration of 100mg / mL sample 10L, store in vials and injection needles (see Table 3B for material information), fill carbon ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com