Pharmaceutical composition of diclofenac sodium for injection and preparation method thereof
A technology of diclofenac sodium and its composition, which is applied in the field of medicine, can solve the problems of poor long-term stability of diclofenac sodium preparations, high toxicity and side effects, and easy crystallization and degradation, etc., so as to improve the analgesic effect, reduce the damage to the human body, and reduce the dosage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
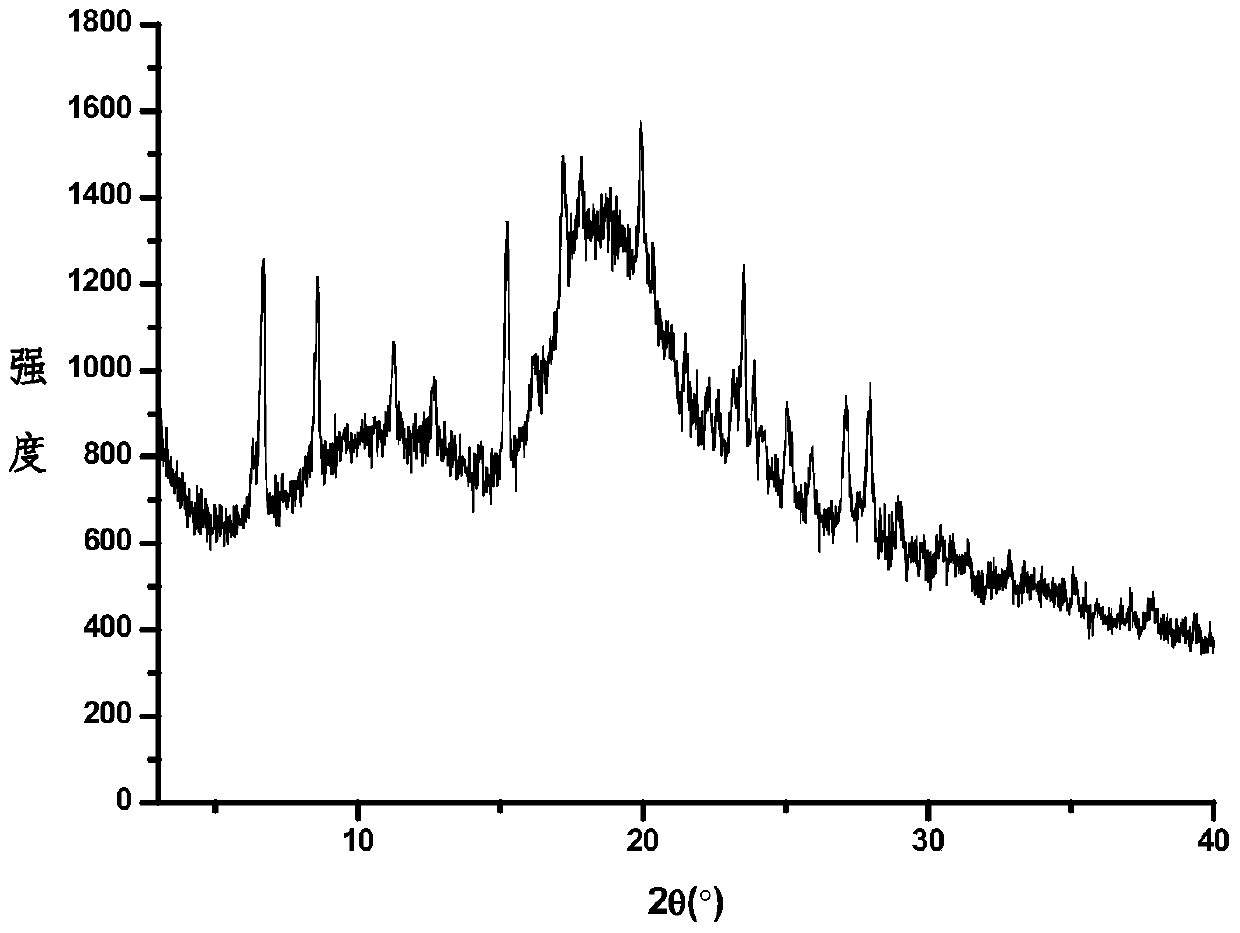
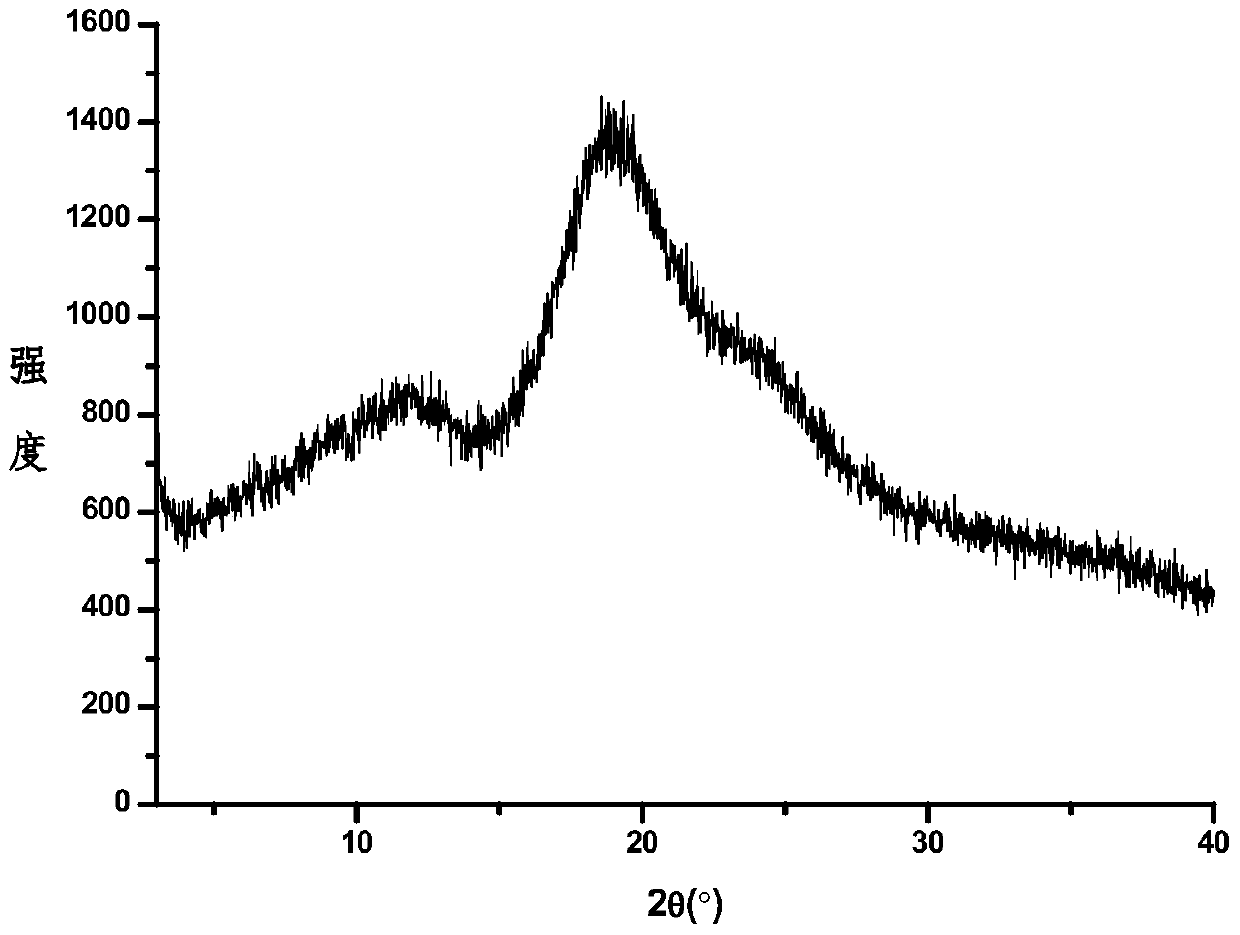
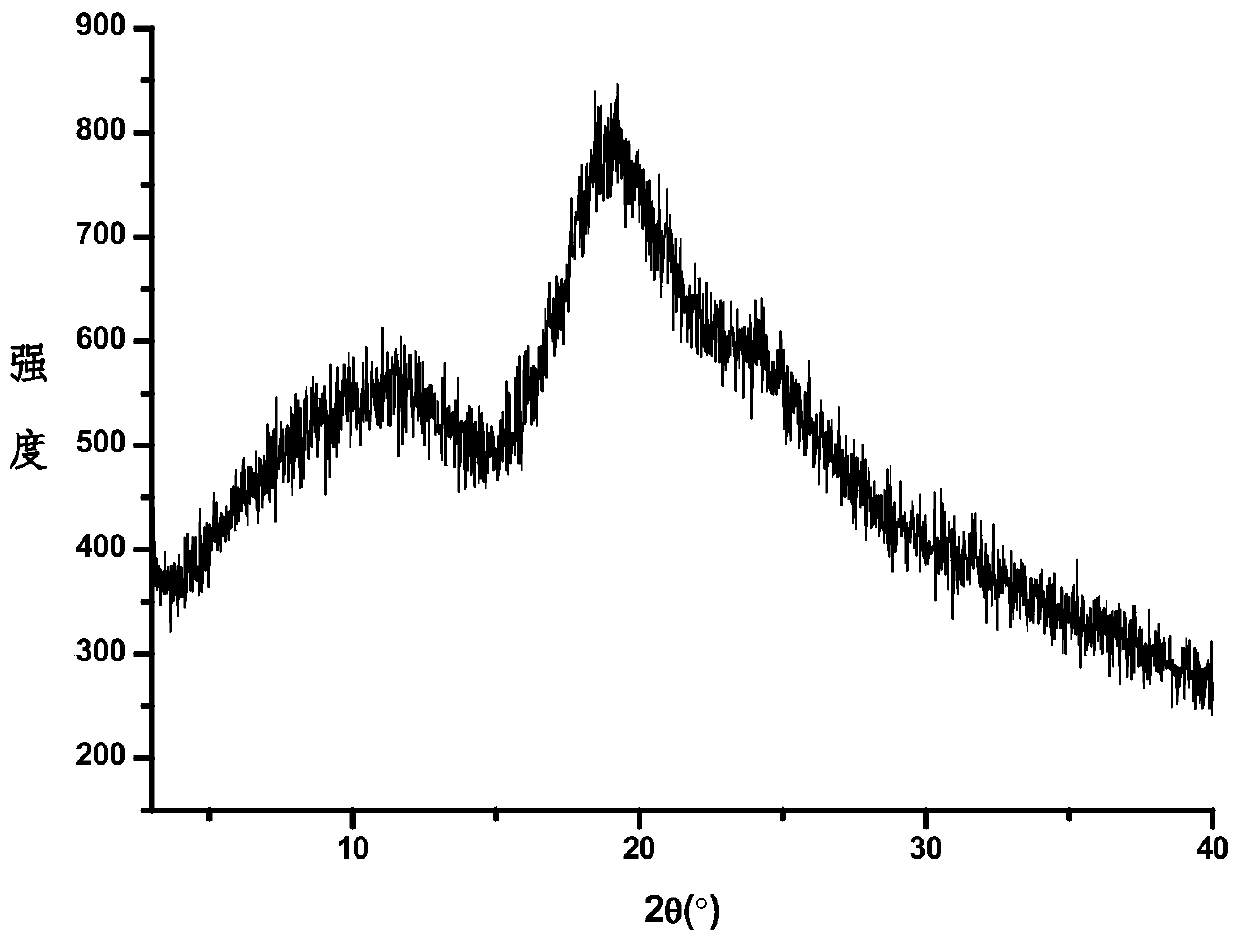
Image
Examples
preparation Embodiment 1
[0057]
[0058] The preparation method is:
[0059] 1) Dissolve hydroxypropyl-β-cyclodextrin in part of the water for injection, then add diclofenac sodium, stir until completely dissolved, add tromethamine and sodium hydroxide, stir to dissolve, and add the remaining amount of water for injection;
[0060] 2) Add 0.1% activated carbon, stir for 15 minutes, decarbonize with a titanium rod filter, and then sterilize with a 0.22 μm filter membrane to obtain an intermediate solution;
[0061] 3) detecting the content of diclofenac sodium in the intermediate solution by HPLC;
[0062] 4) Fill the glass bottle with nitrogen, control the residual oxygen amount to 1.7%, and the dissolved oxygen amount to 0.7 mg / L. According to the measurement results of the diclofenac sodium content, the obtained medicinal solution is divided into each glass bottle according to the preparation unit, Half stopper;
[0063] 5) Put the filled glass bottles in a freeze-drying box, cool down to -40°C...
preparation Embodiment 2
[0065]
[0066] The preparation method is:
[0067] 1) Dissolve sulfobutyl-β-cyclodextrin in part of the water for injection, then add diclofenac sodium, stir until completely dissolved, add tromethamine, stir to dissolve, and add the remaining amount of water for injection;
[0068] 2) Add 0.05% activated carbon, stir for 20 minutes, decarbonize with a titanium rod filter, and then sterilize with a 0.22 μm filter membrane to obtain an intermediate solution;
[0069] 3) detecting the content of diclofenac sodium in the intermediate solution by HPLC;
[0070] 4) Fill the glass bottle with nitrogen, control the residual oxygen amount to 1.6%, and the dissolved oxygen amount to 0.6 mg / L. According to the measurement results of the diclofenac sodium content, the obtained medicinal solution is divided into each glass bottle according to the preparation unit, Half stopper;
[0071] 5) Put the filled glass bottles in a freeze-drying box, cool down to -40°C, pre-freeze at -40°C f...
preparation Embodiment 3
[0073]
[0074] The preparation method is:
[0075] 1) Dissolve glucosyl-β-cyclodextrin in part of the water for injection, then add diclofenac sodium, stir until completely dissolved, add disodium hydrogen phosphate, stir to dissolve, and add the remaining amount of water for injection;
[0076] 2) Add 0.05% activated carbon, stir for 30 minutes, decarbonize with a titanium rod filter, and then sterilize with a 0.22 μm filter membrane to obtain an intermediate solution;
[0077] 3) detecting the content of diclofenac sodium in the intermediate solution by HPLC;
[0078] 4) Fill the glass bottle with nitrogen, control the amount of residual oxygen to 1.5%, and the amount of dissolved oxygen to be 0.5 mg / L. According to the measurement results of the content of diclofenac sodium, the obtained medicinal solution is divided into each glass bottle according to the preparation unit, Half stopper;
[0079] 5) Put the filled glass bottle in a freeze-drying box, cool down to -40°C,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com