Bicyclol-based derivative, preparation and applications thereof
A derivative, bicyclic alcohol technology, applied in the field of medicinal chemistry, to achieve the effect of improving the pathological damage of liver tissue, improving the survival rate of liver cells, and prolonging the survival time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The synthesis of embodiment 1 compound WLP-S-17
[0036]
[0037] a. SOCl 2 / DMF; b.TEA / CH 2 Cl 2 or CH 3 COCH 3 ; c.CH 3 SO 3 H / CH 3 Oh
[0038] 5.1g (13.1mmol) of compound 1 (i.e., bicyclic alcohol) was placed in a 100ml three-neck flask with magnetic stirring and a thermometer, and 50ml of dry DMF was added to dissolve the solid completely. After cooling to 0°C in an ice bath, slowly add 4.5ml ( 61.8mmol) of SOCl 2 , control the system temperature not to exceed 5°C. After the dropwise addition, the mixture was reacted in an ice bath for 30 min until TLC showed that the reaction of the raw materials was complete. Pour the reaction system into about 100 g of crushed ice, stir well, a large amount of white solid precipitates out, filter it, wash the filter cake with a small amount of distilled water and diethyl ether successively, and drain it. Air-dried and weighed to obtain a total of 4.9 g of white solid (compound 2), yield: 91.7%. MS-FAB: [M+2H]+=410....
experiment example 1
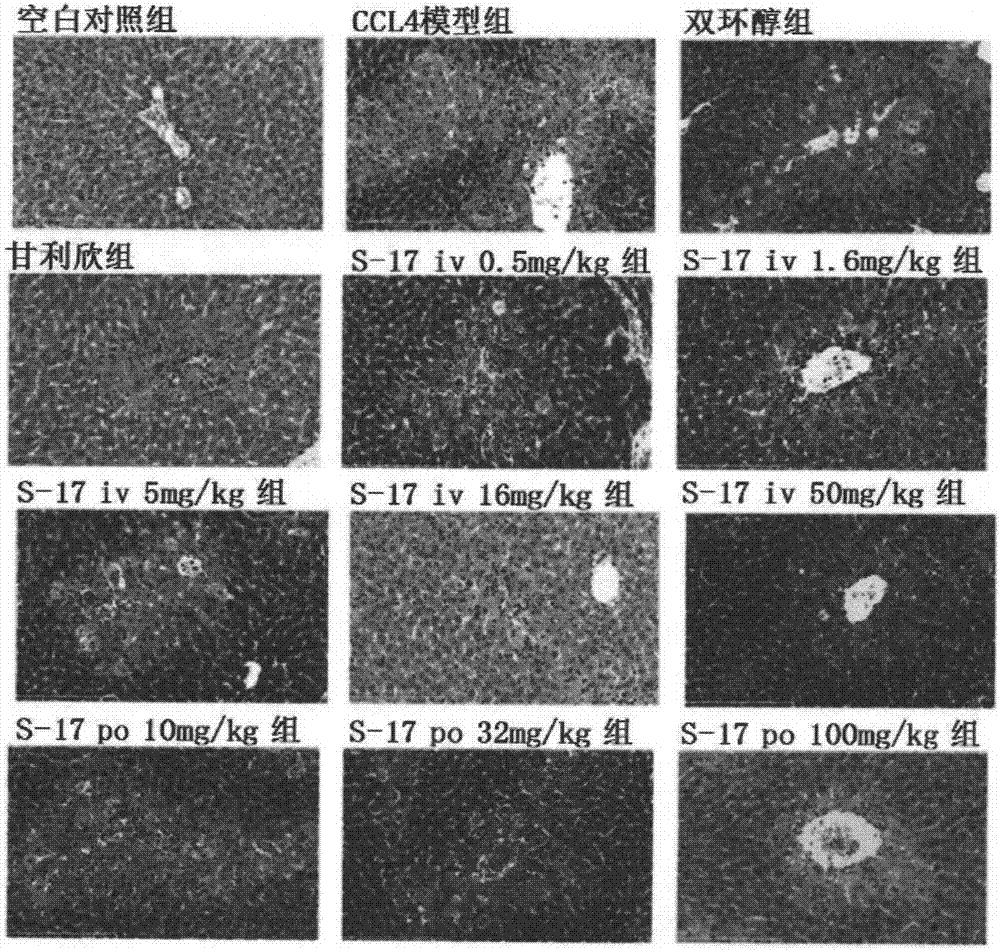
[0042] Experimental example 1 utilizes CCl 4 Induced mouse acute liver injury model detects embodiment compounds
[0043] 1 CCl 4 Establishment of induced acute liver injury model in mice and administration method
[0044] SPF grade male ICR mice (20-22g), were randomly divided into 14 groups after adapting to the environment, blank control group, CCl 4 Model group, WLP-S-17 tail vein 0.5mg / kg group, WLP-S-17 tail vein 1.6mg / kg group, WLP-S-17 tail vein 5mg / kg group, WLP-S-17 tail vein 16mg / kg group kg group, WLP-S-17 tail vein 50mg / kg group, WLP-S-17 oral 1mg / kg group, WLP-S-17 oral 3.2mg / kg group, WLP-S-17 oral 10mg / kg group, WLP - S-17 oral administration 32mg / kg group, WLP-S-17 oral administration 100mg / kg group, bicyclol 200mg / kg group and Ganlixin 100mg / kg group, 8-10 rats in each group. Each tail vein administration group of WLP-S-17 and Ganlixin injection group started tail vein administration 3 days before modeling, and administered once a day for a total of 3 day...
experiment example 2
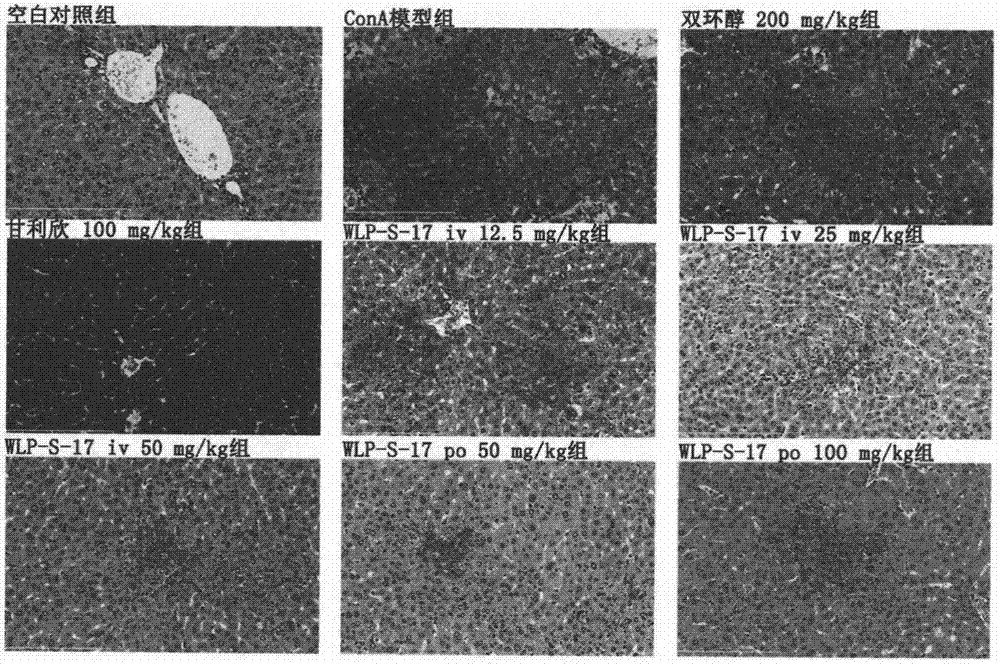
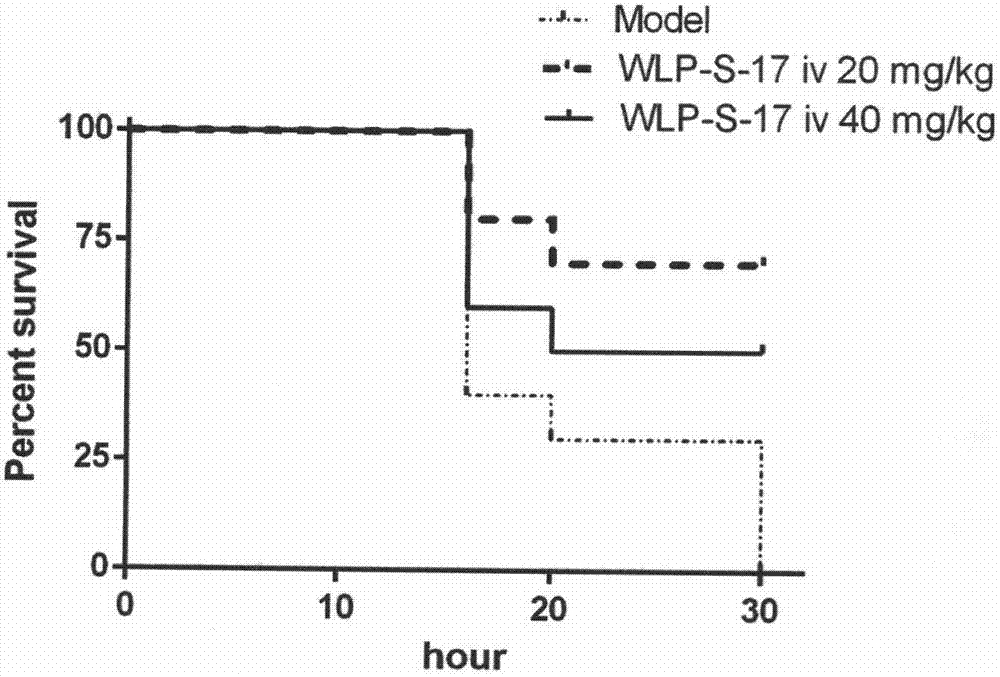
[0077] Experimental Example 2 Using the ConA-induced acute immune liver injury model in mice to detect the compounds of the examples
[0078] 1. Establishment of ConA-induced acute immune liver injury model in mice and administration method
[0079] SPF male ICR mice (20-22g) were randomly divided into 9 groups after adapting to the environment, blank control group, model group, WLP-S-17 100mg / kg orally administered group, WLP-S-17 50mg / kg orally administered Group, WLP-S-17 tail vein 50mg / kg group, WLP-S-17 tail vein 25mg / kg group, WLP-S-17 tail vein 12.5mg / kg group, positive control drug bicyclol 200mg / kg group, positive Control drug Ganlixin 100mg / kg group. There are 9-10 animals in each group. Each tail vein administration group of WLP-S-17 and Ganlixin injection group started tail vein administration 3 days before modeling, and administered once a day for a total of 3 days. Bicyclol group and WLP-S-17 The intragastric administration group was intragastrically administe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com