A functional polyamide monomer, functional polyamide and preparation method
A polyamide monomer and polyamide technology, which is applied in the field of biomass-based polymer materials, can solve the problems of poor mechanical properties of elastomers and unsatisfactory mechanical properties, and achieve an excellent effect of aggregated structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0041] Embodiment 1-1 functional polyamide monomer 1 (R 1 For the preparation of -OH)
[0042] Take 100 g of methyl undecylenate and add 1 g of 1,3-diamino-propanol into 4 ml of tetrahydrofuran. After passing argon for half an hour, place it in a 40°C oil bath, add 10ml of sodium methoxide, the solution becomes clear, and react for 20 hours under the reaction conditions of 40°C. The specific reaction equation is as follows:
[0043]
[0044] Finally, a white powdery solid is obtained after purification, and the white powdery solid is a functional polyamide monomer whose side group R1 is -OH, also called a hydroxyl amide monomer.
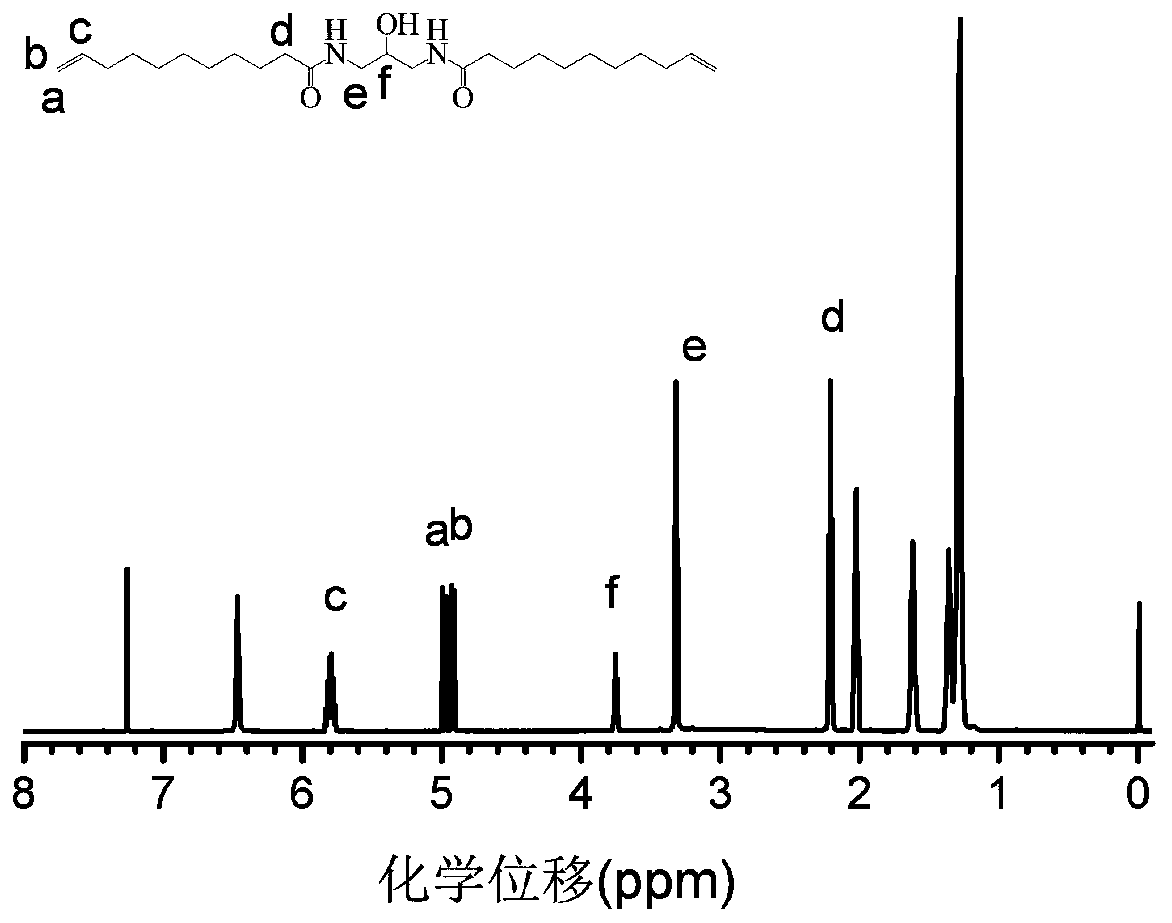
[0045] The 1H NMR spectrum of the functional polyamide monomer 1 prepared in this embodiment is as follows figure 1 As shown, the purity of the obtained functional polyamide monomer 1 is very high. From figure 1 Peak e at 3.38ppm and peak f at 3.8 can be seen that 1,3-diamino-propanol has been successfully integrated into the monomer, and then...
Embodiment 1-2
[0046] Embodiment 1-2 functional polyamide monomer 1 (R 1 For the preparation of -OH)
[0047]1 weight part of undecylenic acid methyl ester and 1 weight part of 1,3-diamino-propanol were added to 10 weight parts of tetrahydrofuran. After passing argon for half an hour, place it in an oil bath at 40°C, add 10 -4 After parts by weight of sodium methoxide, the solution becomes clear, reacted for 2 hours under the reaction condition of 40° C., and finally obtained a white powdery solid through recrystallization.
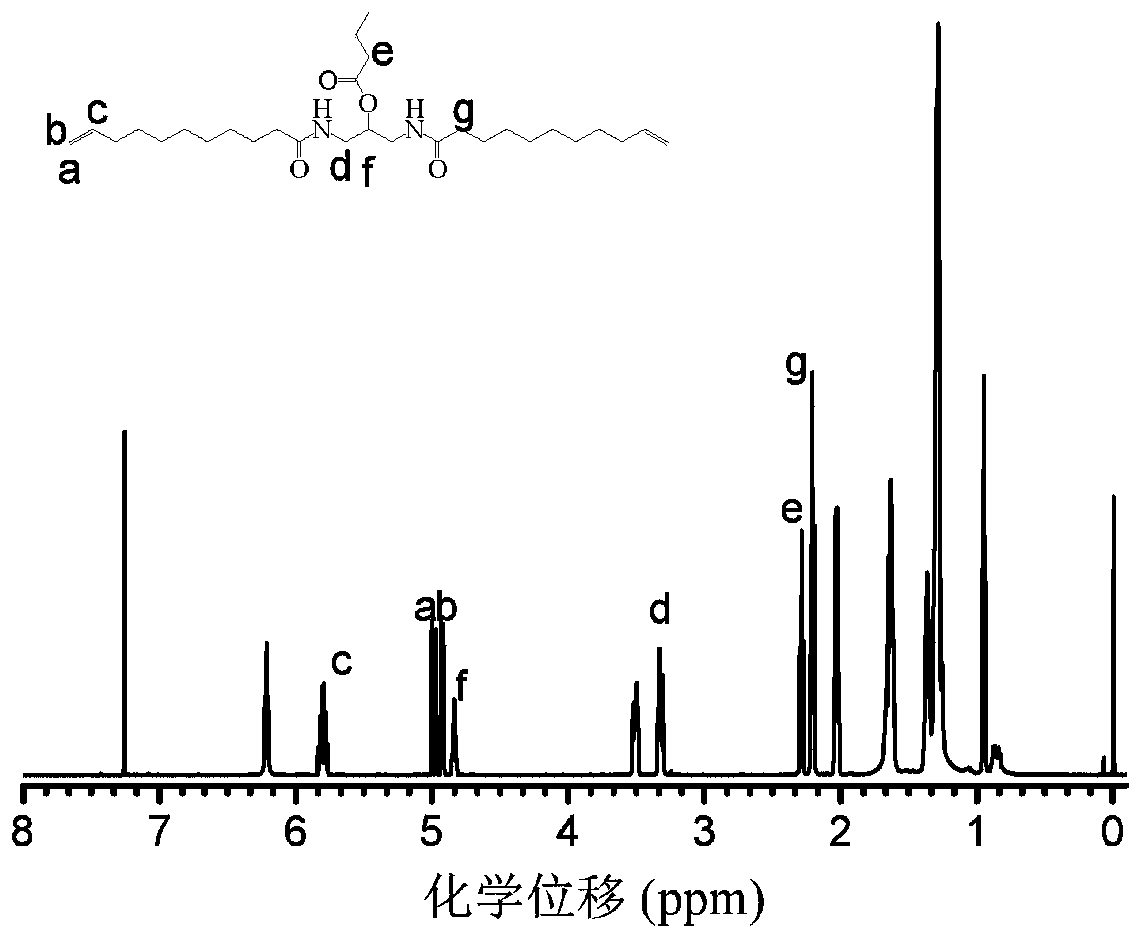
[0048] The 1H NMR spectrum of the functional polyamide monomer 1 prepared in this example also shows a high purity.
Embodiment 1-3
[0049] Embodiment 1-3 functional polyamide monomer 1 (R 1 For the preparation of -OH)
[0050] Take 1000 parts by weight of undecylenic acid methyl ester, and add 10 parts by weight of 1,3-diamino-propanol into 1000 parts by weight of tetrahydrofuran. After passing argon for half an hour, place it in an oil bath at 40°C, add 10 parts by weight of sodium methoxide, the solution becomes clear, react for 36 hours at 120°C, and finally obtain a white powdery solid through recrystallization.
[0051] The 1H NMR spectrum of the functional polyamide monomer 1 prepared in this example also shows a high purity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| elastic modulus | aaaaa | aaaaa |
| breaking strength | aaaaa | aaaaa |
| elongation at break | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com