D-pi-A dipyrro fluorescent dye and synthesis method thereof
A technology of dipolypyrrole and compound, which is applied in the directions of organic dyes, luminescent materials, chemical instruments and methods, etc., can solve the problems of difficult expansion of substrates, long reaction time, complicated reaction process, etc., and achieves easy control and saving of synthetic reaction conditions. Production time, the effect of simplifying the reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
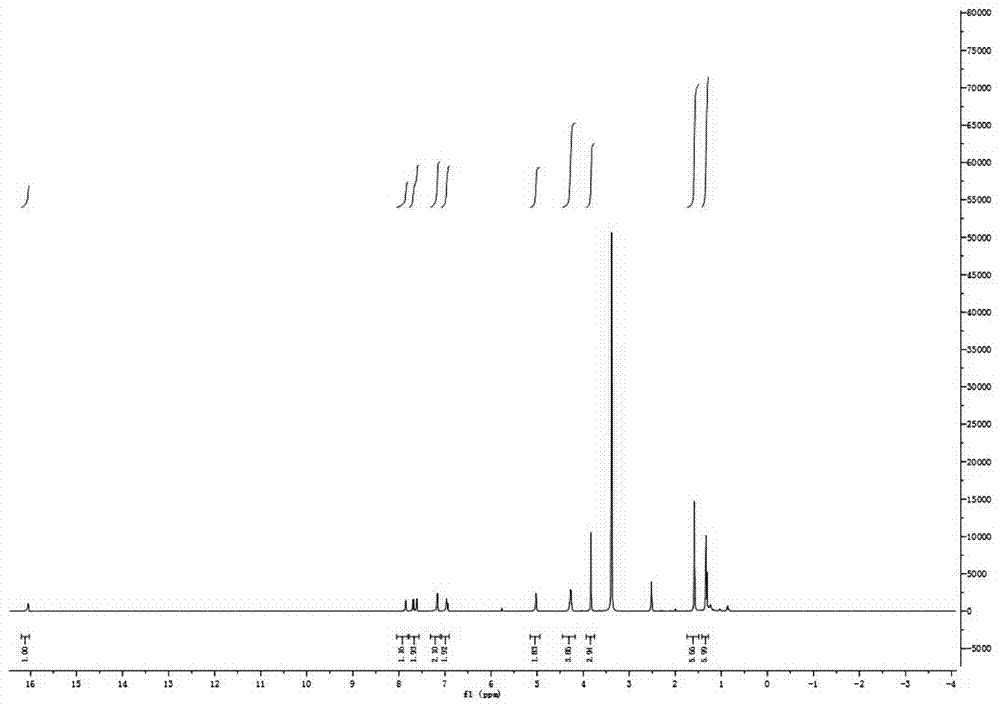
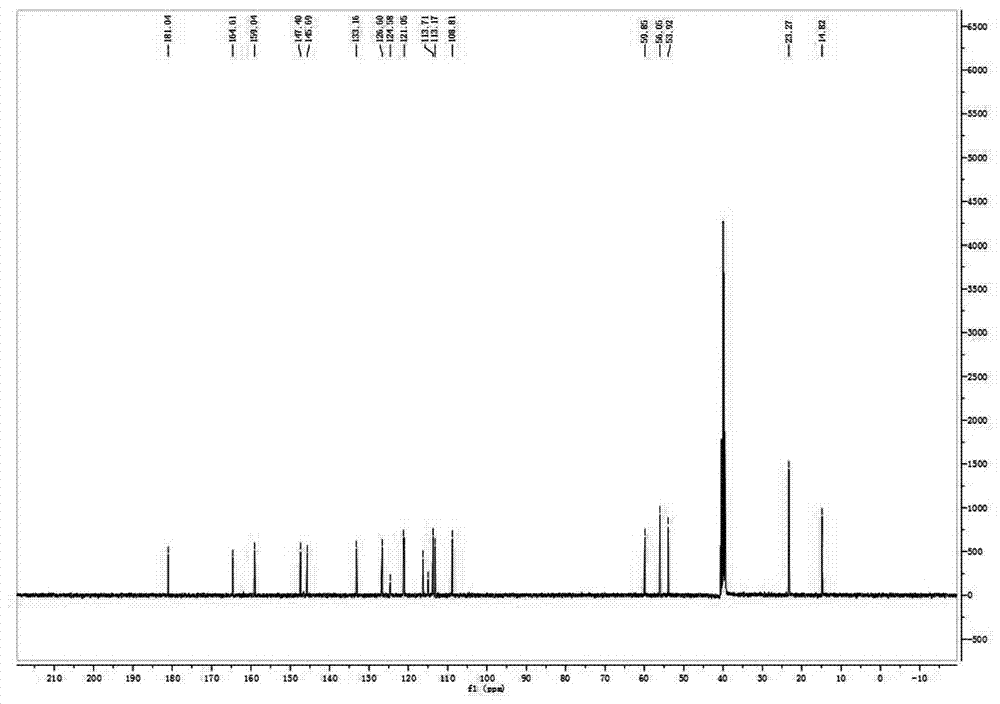
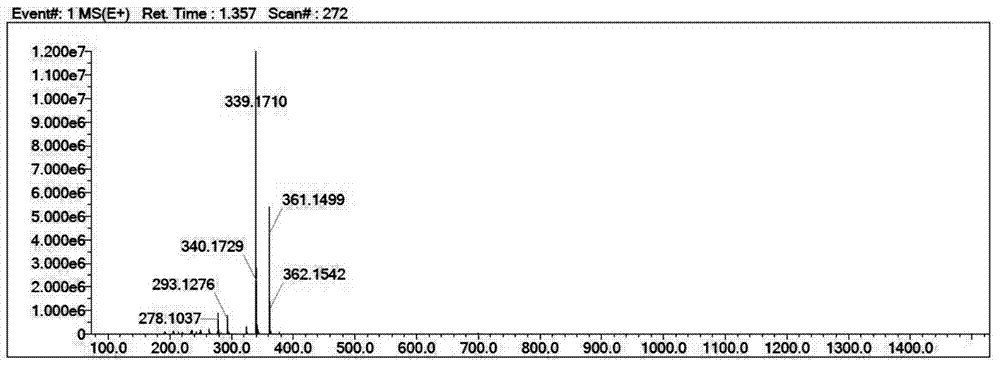
[0036] Take 2,3,3-trimethyl-6-methoxyindole 1 (1.89g, 9.00mmol), weigh 2-formylpyrrole (1.72g, 18.00mmol), measure 0.15L of toluene solution, and take 0.24 mL of glacial acetic acid and 0.30 mL of piperidine were mixed, heated and stirred, refluxed at 120°C for 2 hours, spin-dried and purified by filtration to obtain yellow compound 3 (949.60 mg), yield: 31%.
[0037]
Embodiment 2
[0039] Take 2,3,3-trimethyl-6-methoxyindole 1 (18.00mmol), weigh 2-formylpyrrole (18.00mmol), measure 0.15L of toluene solution, take 0.24mL of glacial acetic acid, piper 0.30 mL of pyridine was mixed, heated and stirred, refluxed at 120° C. for 2 hours, spin-dried and purified by filtration to obtain yellow compound 3 (919.60 mg), yield: 35%.
[0040]
Embodiment 3
[0042] Take 2,3,3-trimethyl-6-methoxyindole 1 (9.00mmol), weigh 2-formylpyrrole (36.00mmol), measure 0.15L of toluene solution, take 0.24mL of glacial acetic acid, piper 0.30 mL of pyridine was mixed, heated and stirred, refluxed at 120°C for 2 hours, spin-dried and purified by filtration to obtain yellow compound 3 (900.60 mg), yield: 42%:
[0043]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com