3,4-difluoroacetophenone substituted viologen compound and preparation method and application thereof
A technology for difluoroacetophenone and compound, which is applied in 3, can solve the problems of harsh conditions for viologen discoloration, the substance detection line needs to be improved, and the color development period of the compound is short, so as to achieve simple operation, high yield and easy purification. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
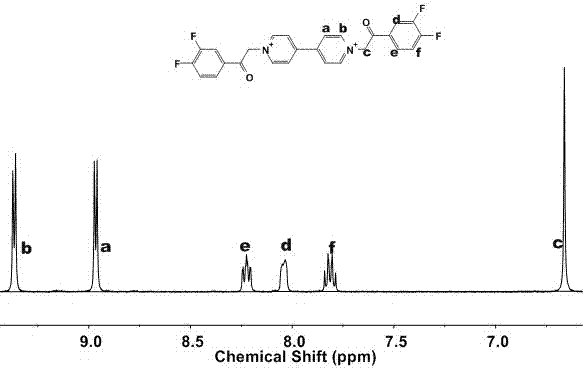
[0045] Embodiment one: the synthetic acetophenone substituent novel viologen adopts the following steps:
[0046] ① Weigh 4,4'-bipyridine (1.56g, 10mmol) and 4-chloroacetyl-1,2-difluorobenzene (4.66g, 25mmol) in a 50mL single-necked round-bottomed flask, and use anhydrous DMF (15mL) dissolve. The solution was refluxed at 120°C for 36 hours, during which a light yellow precipitate was formed. After the reaction was completed, cool to room temperature, centrifuge to obtain a precipitate, and then wash with anhydrous DMF and acetone for 3 to 5 times, until the washing liquid turns from brown to almost colorless, and the crude product is obtained.
[0047] ②At room temperature, add high-purity water to the crude product drop by drop until completely dissolved. After adding a large amount of acetone to the solution, a large amount of light white precipitates precipitated out. After the precipitate was centrifuged and dried in vacuum for 8 hours, the obtained product was 3.55 g (...
Embodiment 2
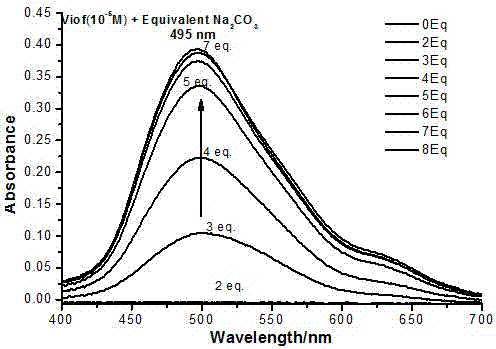
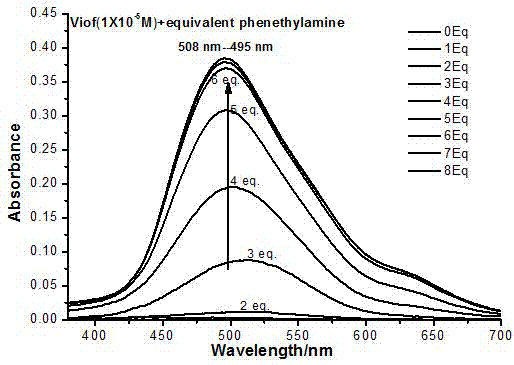
[0048] Embodiment 2: Alkali titration 3,4-difluoroacetophenone substituent novel viologen adopts the following steps:
[0049] First prepare 1×10 of 3,4-difluoroacetophenone substituent viologen -5 and 5×10 -3 Aqueous solution of M, then NaOH, phenethylamine, diethylamine, diisopropylamine, Na 2 CO 3 , piperazine, triethanolamine, triethylenetetramine and triethylamine were titrated with different equivalents, and the solution after titration was tested for ultraviolet-visible absorption spectrum. In the alkali titration process, the phenomenon is almost the same, with the increase of the amount of alkali, the solution changes from colorless to pink to red.
Embodiment 3
[0050] Embodiment 3: The following steps are used to make the temperature-sensitive test paper of the new viologen substituent of 3,4-difluoroacetophenone:
[0051] First prepare 1×10 of 3,4-difluoroacetophenone substituent viologen -2 ~1×10 -3 M aqueous solution, and then soak the clean test paper in the solution, take it out, put the test paper in the natural environment to dry, and obtain the viologen temperature and acid-base gas sensing test paper. The color of the test paper changes from white to purple as the temperature increases and the purple color deepens as the temperature increases. The test paper for NH 3 Gas is very sensitive, in case of NH 3 The color changed to blue-purple, and the discolored test paper was treated with HCl gas to become white. The test paper can be used for temperature and acid-base gas (such as NH 3 ) sensing is reversible.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com